NIH provides $9.8 million in funding for new clinical trial using Advantage Arrest Silver Diamine Fluoride 38%
Advantage Silver Dental Arrest LLC (Redmond, Oregon) and Elevate Oral Care LLC (West Palm Beach, Florida) announced today that the National Institute of Dental and Craniofacial Research (NIDCR) of the National Institutes of Health (NIH) has provided $9.8 million to fund a Phase III randomized clinical trial using Advantage Arrest Silver Diamine Fluoride 38% for arresting caries in children.
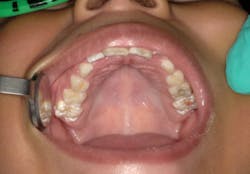
Advantage Arrest was cleared as a medical device in 2014 for the treatment of dentinal hypersensitivity, the same clearance given to 2.5% and 5% sodium fluoride varnish. It is being used off-label in the US for arresting and preventing caries in children and adults.
In 2016, Advantage Arrest was the first oral care product to receive the FDA’s Breakthrough Therapy Designation (BTD) for the arrest of tooth decay in children and adults. BTD represents the FDA’s effort to address an unmet, serious, and life-threatening medical need where there is no available therapy.
In use for more than 50 years as a caries-arresting drug in Japan and other countries, the silver diamine fluoride (SDF) designation was granted based on the FDA review of 10 worldwide randomized clinical trials evaluating silver diamine fluoride for caries arrest in children aged 3 to 9 or adults aged 60 to 89, as well as studies conducted by Advantage Silver Dental Arrest LLC. Collectively these trials involved approximately 1,500 subjects who were monitored for 1 to 3 years.
The American Dental Association added a new Code on Dental Procedures and Nomenclature (CDT Code D1354, Interim Caries Arresting Medicament Application) to achieve uniformity, consistency, and specificity in documenting SDF treatment. Insurers and certain state Medicaid programs are reimbursing for the cost of the therapy for caries arrest.
Recommended:
The dos and don'ts of silver diamine fluoride | By Pamela Maragliano-Muniz, DMD, and Brian Nový, DDS
“The NIDCR funding represents another major milestone in our over nine-year effort spearheaded by Dr. Peter Milgrom of Advantage Silver Dental Arrest and the University of Washington Dental School," said Mike Shirtcliff, DMD, president of Advantage Silver Dental Arrest. "Knowing how this option continues to improve the treatment of dental caries, not just with patients in low socioeconomic circumstances, but for numerous Americans across all age and economic spectrums, has been extremely gratifying for me as a health professional.”
“We are proud to be a part of helping to significantly improve the oral health of so many Americans in a cost-effective manner not previously available in the US," said Kevin Thomas, president of Elevate Oral Care, the commercialization organization for Advantage Arrest. "Every day we hear more stories from dental professionals about how Advantage Arrest is not only helping so many of their patients, but also how it is improving their practices by providing an effective and efficient way to treat this serious and rampant disease.”
The Phase III clinical trial will provide the necessary data required by the FDA for approving a cavity-arrest drug claim in the US. It will follow 1,060 children ages 2 through 5 in preschool programs such as Head Start during a school year. The principal investigator is Margherita Fontana, DDS, PhD, a professor in the Department of Cariology, Restorative Sciences and Endodontics at the University of Michigan. Researchers from the University of Iowa, New York University, Indiana University, University of Otago in New Zealand, University of Hong Kong, and University of Baltimore will also participate in this multicenter study.
Editor's note: This article is an edited version of a press release issued 10 October 2017 by Advantage Silver Dental Arrest LLC and Elevate Oral Care LLC.
This article first appeared in the Apex360 e-newsletter. Apex360 is a DentistryIQ partner publication for dental practitioners and members of the dental industry. Its goal is to provide timely dental information and present it in meaningful context, empowering those in the dental space to make better business decisions.
Subscribe to the Apex360 e-newsletter here.
Dental news and press releases may be sent to Apex360 editors at dentalpress@pennwell.com">dentalpress@pennwell.com.