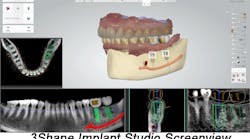
3Shape Implant Studio receives FDA 510(k) market clearance
Competition in the dental imaging market continues to heat up. 3Shape, maker of digital 3-D solutions for dental labs and dental clinics, announced today that the U.S. Food and Drug Administration (FDA) has granted 510(k) market clearance for the sale of its Implant Studio software in the U.S.
3Shape Implant Studio is used widely in Asia, Europe and South America. The software merges CT/ Cone Beam CT imagingwith 3-D digital surface scans of the teeth and gingival situation to provide a digital view of dental patients. The software then enables dental professionals to evaluate the clinical situation, including bone density and nerve positions, for creating prosthetic implant planning and surgical guide design.
The dental implant market in the U.S. is projected to reach $5 billion by 2018. Three million Americans already have implants, and that number is predicted to grow by 500,000 per year according to the American Academy of Implant Dentistry.
“3Shape is very excited about this FDA 510(k) market clearance, which opens the door to new service options for both clinics and labs in North America," said Flemming Thorup, 3Shape’s President and CEO. "Importantly, as more and more people are choosing dental implants to meet their restoration needs, Implant Studio will enable dental professionals to more effectively care for them.”
Implant Studio will be available through 3Shape resellers early Q1 2015. Availability to end-users will depend on the specific system configuration.
3Shape experts will be demonstrating Implant Studio, including complete workflows using intraoral and CBCT scans, at the 2014 Greater NY Dental Meeting, November 30 - December 3, 3Shape booth 424.
Related Articles
3Shape releases Dental System 2014