WRITTEN BY
Janet Hatcher Rice, DDS
Today's choice for tomorrow's standard of care
The challenge of translating dental research into today's clinical practice can only be met by balancing the art of patient care with a sound scientific base. This is especially true with lasers, balancing the urgent need for learning about these revolutionary devices with their clinical applications and selection in delivering quality patient care. This article will review 40 years of laser research on enamel and dentin, and discuss the biophysics behind laser technology. It will also present several clinical applications for the erbium laser and explore its impact on improved patient care.
The Er:YAG laser obtained clearance for marketing for use on enamel and dentin by the Food and Drug Administration (FDA) in 1997. During these past six years, Er:YAG and Er,Cr:YSGG have obtained FDA clearance for several oral uses, including removal of caries, enamel, dentin, cement, composite, and glass ionomer. This technology is becoming the standard of care for many cavity preparations and the treatment of choice by many patients. It is imperative for the practitioner to obtain accurate information and apply it appropriately. In 1780, Abigail Smith Adams wrote to her husband, America's second President, "Learning is not attained by chance. It must be sought for with ardor and attended to with diligence."
Historical review of laser use on enamel and dentin
In 1960, shortly after the invention of the ruby laser by Maiman1 at Hughes Research Laboratories, research began on laser applications for teeth. Goldman,2 a physician at the University of Cincinnati, documented in-vitro use of the ruby laser on dental tissues. He, along with Stern and Sognnaes,3 assessed the ability of the laser for removal of oral hard tissue. Although the research was not favorable, they laid the foundation for all future studies by examining the basic parameters of safety and effectiveness. Their research demonstrated that the short 694 nm wavelength of ruby lasers was found to increase the likelihood of pulpal trauma, due to thermal radiation.4 The ruby was abandoned by the end of the decade. In the 1970s, research on new laser wavelengths, such as Neodymium (Nd) and carbon dioxide (CO2), produced successful outcomes in "heat treating" enamel to decrease permeability to acidic fluids.5,6,7
By the early 1980s, research on hard-tissue applications for the dental laser languished, with research focused on soft-tissue oral surgery. The work of Myers and Myers8,9,10 in the mid-1980s spurred interest in hard-tissue applications of the Nd:YAG; by the early 1990s, this wavelength (1064 nm) was found to be useful for minor hard-tissue removal. The search continued to find a wavelength that was well-absorbed in hydroxyapatite, without penetration or damage to pulpal tissue.
In 1988, Paghdiwala11 evaluated the erbium Er:YAG laser for ablation of dental hard tissues, or removal by vaporization, in the United States. Tests demonstrated that successful "drilling" of holes in enamel and dentin, without water, did not produce cracking, charring, or significant temperature rise in the pulp. Hibst and Keller12 assessed the Er:YAG laser for selective ablation of caries with negligible effects on adjacent hard and soft tissues. Glockner et al13 reported that tooth temperatures actually decreased as a result of cooling with air and water. Wigdor et al14,15 found uniform dentinal tubules without debris in laser-prepared cavities, similar to a high-speed rotary handpiece. Histologically, the normal architecture of the odontoblastic cellular layer was found to be retained, with no irreversible inflammatory cell infiltration. Frentzen et al15 demonstrated that surface morphology of enamel remained rough, allowing for enhanced microretention. Aoki et al16 reported the effectiveness of the Er:YAG laser for removal of root caries without thermal damage to the tooth. Fazlur et al17 investigated microleakage; scanning electron microscopy (SEM) revealed good adaptation of composite resin and glass ionomer in cavities prepared with lasers and restored with composite resin or glass ionomer. M. Hossain et al18 also investigated microleakage; SEM revealed decreased microleakage after Er:YAG laser irradiation. The time required for cavity preparation was also assessed. When compared to the dental air turbine/bur drill, the Er,Cr:YSGG was shown to be equivalent in assuring efficient, effective, and safe cavity preparation with retention of restorations. This was demonstrated in a randomized clinical study by J. Hadley, D.A. Young, et al.19
Research continues, extending the application of erbium lasers to procedures with bone and cementum. This will be discussed in future WDJ issues.
Erbium terminology
The term laser is an acronym for light amplification by stimulated emission of radiation. Erbium lasers are categorized in the mid-infrared range of the electromagnetic spectrum, with light emitted as invisible, nonionizing, thermal radiation.
Currently, two types of erbium lasers are available, each emitting a unique wavelength, depending on the material present in the laser rod inside the device. The rods contain the active medium, host crystals, into which dopant atoms are uniformly distributed. For example, Er:YAG laser devices consist of a yttrium aluminum garnet (YAG) host crystal, doped with erbium ions. These generate a wavelength of 2936 nm, while the yttrium scandium gallium garnet (YSGG) host crystal, doped with chromium-sensitized erbium, emits a wavelength of 2790 nm. Wavelength is a major factor in the absorption of the laser light by biologic tissue.
Biophysics of erbium
According to current science, laser light energy must be converted into some other form of energy to produce a biologic effect. Both types of erbium lasers are categorized as laser light that is converted into acoustic (mechanical) energy. This type of energy is in the form of a shock wave, physically disrupting the target tissue. Although wavelength is a major factor, laser peak power, pulse duration, pulse energy, and beam focusing are critical parameters.20
Production of acoustic shock waves is due to the rapid, volumetric expansion occurring when water changes from liquid to gas. This expansion causes the surrounding tooth structure to explode, known as spallation. The water spray of the handpiece accelerates this effect by removing exploded tissue, transferring minimal heat to the remaining tooth.21
null
null
null
null
Morphology of erbium laser preparations
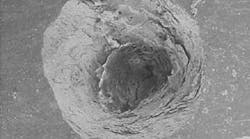
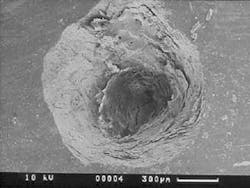
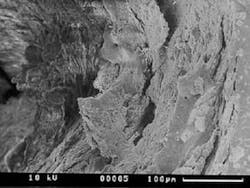
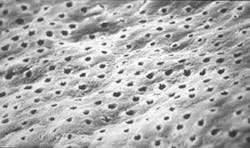
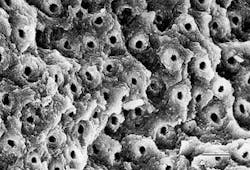
The structural morphology of enamel irradiated with erbium laser light is an ablated area with surface roughness and a white, chalky appearance called enamel modification. SEM pulsing action creates a surface effect that resembles chemical etching, with expanded surface area and increased surface tension (Figure 1). Microretention in undercuts and rough surfaces enhances the strength of bonding agents (Figure 2). When acid etch and laser modification are combined, the bond strength of restorative resins is greater than either method alone.22 The dentin has open tubules and no smear layer, also ideal for composite resin bonding (Figure 3).
Clinical description of erbium use
1. Begin by using the laser manufacturer's recommended power settings, ANSI23 safety guidelines, and universal precautions. Start with the least amount of power for the desired clinical result.
2. Test the laser outside the mouth before use. The patient can experience the popping sound of the laser, while the operator can adjust water coolant and laser performance.
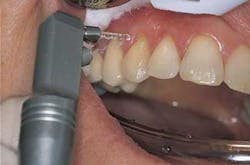
3. Initiate treatment perpendicular to and 1 mm above the surface of the target (Figure 4). If a contact tip is used, lightly touch the target area with the tip. Laser energy is emitted from the tip only, not the sides like a dental bur.
4. Look for the distinct, circumferential marks when the laser removes tooth structure. Some listen for the amplitude of the "pop" that is created by this removal, adjusting the power depending on the intended outcome.
5. Direct the water stream to the target tissue, keeping the operative site wet.
6. Use high-volume evacuation to facilitate cooling and removal of debris.
7. Adjust hand movement for laser tooth preparations, because the traditional hand motions used with high-speed handpieces are not efficient or effective. Since lasers are end-cutting devices, they remove tooth slower than a high-speed drill.19
8. Consider joining an educational group. The Academy of Laser Dentistry — www.laser dentistry.org — is the source for information on all lasers used in dentistry, offering clinical proficiency recognition programs based on the Curriculum Guidelines and Standards for Dental Laser Education.24
Case study — Class I preparation, molar Pretreatment —
A. Diagnostic tests —
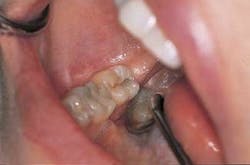
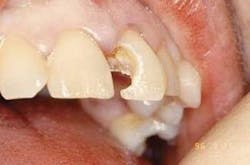
Clinical examination: A 35-year-old female patient presented for an initial oral exam and radiographs. Visual and radiographic exams revealed caries in the occlusal, Tooth No. 31. All other findings were within normal limits.
Dental history: The patient, a nurse, reported a history of unpleasant dental experiences, with poorly defined reaction to local anesthesia. She was investigating treatment options. Her oral health was excellent, except for caries in Tooth No. 31.
Medical history: Unremarkable.
Tooth vitality: Dental history and radiographs revealed no pulpal pathology.
null
null
null
B. Diagnosis and treatment plan —
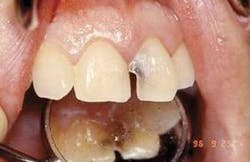
Diagnosis: Occlusal caries Tooth No. 31 (Figure 5).
Treatment plan: Er:YAG laser cavity preparation with selective ablation of caries and enamel modification.
Treatment alternative: Conventional high-speed handpiece and hand instruments for excavation of caries.
Indication: Er:YAG laser provides excellent caries removal and a superior restoration for bonding, without the need for anesthesia in motivated patients.
Contraindications: None.
Informed consent: Obtained.
Treatment —
A. Objective —
To ablate caries, modify enamel, and place a composite restoration.
B. Laser operating parameters —
The Opus 20 (Opusdent, Norwood, Mass.) 2936 nm, pulsed Er:YAG laser, delivered through a flexible hollow waveguide with coaxial air and water was utilized with a 1300µ flat end, noncontact, sapphire tip. The pulse duration of 300 microseconds was constant.
Enamel ablation: 800mj, 8 Hz (6.4 watts), noncontact.
Dentin ablation and caries removal: 500mj, 7 Hz (3.5 watts), noncontact.
C. Treatment sequence —
Enamel ablation: The contact tip was positioned 1 mm above the tooth and at a 90° angle to the enamel. Slow and continuous movement was used, 1 mm up and down and 1 mm laterally. The laser handpiece was started away from the pit and worked inward to achieve perpendicular positioning. Using water and high-speed evacuation, no anesthesia was required (Figure 6).
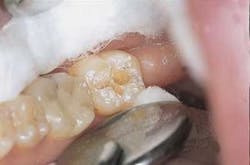
Dentin ablation and caries removal: The tip was rotated just out of contact, using a lateral, less than 1 mm, continuous movement consistent with the rate of removal. The operator repeatedly stopped to visually inspect removal and assess preparation outline, and used hand instruments to verify complete removal of dentin.
Total lasing time: Five minutes.
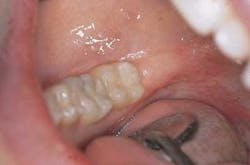
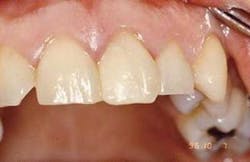
Restoration: Traditional acid-etch technique was utilized and a posterior composite was placed (Figure 7).
Follow-up care —
A. Side effects or complications —
No adverse effects were reported during the procedure or postoperatively. The patient tolerated the procedures well, and no anesthesia was required.
B. Assessment of treatment —
The patient is on continuing care every six months.
C. Long-term results —
Excellent.
D. Healing assessment —
The treatment objective was shown to be effective and the composite restorations are healthy. The tooth remains vital with no sensitivity.
Summary
Based on more than two decades of targeted research, the Er:YAG dental laser is now being used for hard-tissue applications in clinical practice with predictable outcomes. The use of erbium lasers on enamel and dentin has been documented to prepare cavities without charring, carbonization, or cracking and to enhance bond strength when combining laser and acid etch. It can decrease microleakage of composite restorations, and may increase resistance of tooth mineral to dissolution by acid. Laser use should be preceded by adequate training of the dental team, outlined in the Curriculum Guidelines and Standards for Dental Laser Education and adopted by the Academy of Laser Dentistry.24 Continual learning "with ardor and diligence" will be imperative as this technology evolves. Only then can success be achieved in meeting the challenge of relating hard science to the art of patient care.
null
null
null
null
null
null
References
1 Maiman TH: Stimulated optical radiation in ruby. Nature 1960; 187:493-494.
2 Goldman L, Gray JA, Goldman J, et al: Effects of laser impacts on teeth. J Am Dent Assoc 1965; 70:601-606.
3 Stern RH, Sognnaes RF: Laser beam on dental hard tissues. J Dent Res 1964; 43:873-876.
4 Stern KH, Weise EL: High temperature properties and decomposition of inorganic salts: Part 2. Carbonates. In National Standard Reference Data Series, Vol. 30. National Bureau of Standards, US Government, 1969.
5 Yamamoto H, Ohabe H, Ooya K, et al: Laser effect on vital oral tissues: A preliminary investigation. J Oral Pathol 1972; 1:256-264.
6 Yamamoto H, Ooya K: Potential of yttrium-aluminium-garnet laser in caries prevention. J Oral Pathol 1974; 38:7-15.
7 Yamamoto H, Sato K: Prevention of dental caries by Nd:YAG laser irradiation. J Dent Res 1980; S9:1271-1277.
8 Myers TD, Myers WD: In vitro caries removal. J Calif Dent Assoc 1988; 16:9-11.
9 Myers TD, Myers WD: In vivo caries removal utilizing the Nd:YAG laser. J Mich Dent Assoc 1985; 67:66-69.
10 Myers TD, Myers WD: The use of a laser for debridement of incipient caries. J Prosthet Dent 1985; 53:776-779.
11 Paghdiwala AF: Application of the Erbium: YAG laser in hard dental tissues: Measurement of the temperature changes and depths of cut. In: Profio AE (ed): Laser Research in Medicine, Dentistry and Surgery, ICALEO'88. Santa Clara, CA, Laser Institute of America 1988; 192-201.
12 Keller U, Hibst R: Tooth pulp reaction following Er:YAG laser application. In: O'Brien SJ, Dederich DN, Wigdor H. et al (eds): Lasers in Orthopedic, Dental, and Veterinary Medicine. Los Angeles, Proceedings of the International Society for Optical Engineering (SPIE) held in Bellingham, WA 1991; 127-133.
13 Glockner K, Rumpler J, Eveleseder K, et al: Intrapulpal temperature during reparation with the Er:YAG laser compared to the conventional burr: An in-vitro study. J Clin Laser Med Surg 1998; 16:153-157.
14 Wigdor H, Abt E, Ashrafi S, et al: The effect of lasers in dental hard tissues. J Am Dent Assoc 1993; 124:65-70.
15 Frentzen M, Koort HJ: Histological investigation of mid-infrared laser ablation of dental hard tissues. Presented at Third International Congress on Lasers in Dentistry, Salt Lake City, UT, 1992.
16 Aoki A, Ishikawa I, Yamada JT et al: Comparison between the Er:YAG laser and conventional technique for root caries treatment in vitro. J Dent Res 1998; 77:1404-1414.
17 Fazlur M, Rahman K, Kazuo Y, et al: Study of microleakage of Class I cavities prepared by Er:YAG laser using three types of restorative materials. Laser Med Surg 1998; 16:395.
18 Hossain M, et al: A study on surface roughness and microleakage test in cavities prepared by Er:YAG laser irradiation and etched bur cavities. Lasers Med Sci 2003; 18:25-31.
19 Hadley, J, et al: A laser-powered hydrokinetic system for caries removal and cavity preparation. J Am Dent Assoc 2000; 131-(6):777-785.
20 Featherstone, JD: Caries detection and prevention with laser energy. In: Convissar FA, Dent Clin North Am. Philadelphia, PA; Saunders 2000; 44:955-966.
21 Gimbel CB: Hard tissue procedures. In: Convissar FA, Dent Clin North Am. Philadelphia, PA: Saunders 2000; 44:931-948.
22 Parkins F: Lasers in pediatric and adolescent dentistry. In: Convissar RA, Dent Clin North Am. Philadelphia, PA: Saunders 2000; 44:821-830.
23 American National Standard for Safe Use of Lasers. ANSI Z136.1-1993. Orlando, The Laser Institute of America 1993.
24 Academy of Laser Dentistry, 3300 University Drive, Suite 704, Coral Springs, FL USA 33064; www.laserdentistry.org; (877) LASERS6.
Janet Hatcher Rice, DDS
Dr. Rice practices cosmetic and laser dentistry in Bristol, Va./Tenn. She is president-elect of the Academy of Laser Dentistry and serves on its Ethics, Advertising, and Nomination committees. She is a past president of the AAWD. Contact Dr. Rice at [email protected].
Representative stereoscopic photographs of the prepared cavities used in the abstract on Page 24.
null
null
Representative SEM photographs of the dentin surface of prepared cavities used in the abstract on Page xx.
null
null
Many thanks to Dr. Yukio Nakamura for the use of these four photos.