BREAKING NEWS: New drug approved for skin cancer; new NIH fact sheet explains diabetes, prediabetes test
By Maria Perno Goldie, RDH, MS
New drug approved for skin cancer
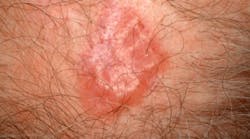
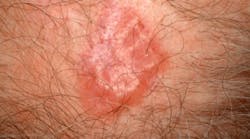
The FDA approved vismodegib (marketed as Erivedge) January 30, 2012, the first drug for the treatment of metastatic basal cell carcinoma. It is indicated for patients whose cancer has advanced locally or metastasized, and for whom surgery or radiation isn't an option.Approval for the once-daily pill was based on a multicenter study of 96 patients with locally advanced or metastatic basal cell carcinoma. Thirty percent of patients with metastatic disease who took vismodegib experienced partial shrinkage of cancerous lesions, and 43% of those with locally advanced disease who took the drug saw partial or complete shrinkage.Vismodegib will have a boxed warning about the potential risk for severe birth defects or death to fetuses. Common treatment side effects include decreased appetite, hair and weight loss, muscle spasms, nausea, and vomiting.
New drug approved for skin cancer
The FDA approved vismodegib (marketed as Erivedge) January 30, 2012, the first drug for the treatment of metastatic basal cell carcinoma. It is indicated for patients whose cancer has advanced locally or metastasized, and for whom surgery or radiation isn't an option.Approval for the once-daily pill was based on a multicenter study of 96 patients with locally advanced or metastatic basal cell carcinoma. Thirty percent of patients with metastatic disease who took vismodegib experienced partial shrinkage of cancerous lesions, and 43% of those with locally advanced disease who took the drug saw partial or complete shrinkage.Vismodegib will have a boxed warning about the potential risk for severe birth defects or death to fetuses. Common treatment side effects include decreased appetite, hair and weight loss, muscle spasms, nausea, and vomiting.
Basal Cell Carcinoma (Credit: skincancer.org)For more information, visit www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm289545.htm.New NIH fact sheet explains test for diabetes, prediabetes
For Immediate Release, Thursday, January 26, 2012 Contact: Mary Harris (301) 496-3583 A new fact sheet from the National Institutes of Health explains the A1C test, a widely used and important test to diagnose type 2 diabetes and prediabetes, and to monitor blood glucose levels of people with type 1 and type 2 diabetes.The A1C blood test provides information about average blood glucose levels, also called blood sugar, over the past three months. The test is sometimes referred to as the hemoglobin A1c, HbA1c, or glycohemoglobin test. The test result is reported as a percentage. The higher the percentage, the higher a person's average blood glucose levels, which can cause complications in people with diabetes. A normal A1C level is below 5.7 percent.
For Immediate Release, Thursday, January 26, 2012 Contact: Mary Harris (301) 496-3583 A new fact sheet from the National Institutes of Health explains the A1C test, a widely used and important test to diagnose type 2 diabetes and prediabetes, and to monitor blood glucose levels of people with type 1 and type 2 diabetes.The A1C blood test provides information about average blood glucose levels, also called blood sugar, over the past three months. The test is sometimes referred to as the hemoglobin A1c, HbA1c, or glycohemoglobin test. The test result is reported as a percentage. The higher the percentage, the higher a person's average blood glucose levels, which can cause complications in people with diabetes. A normal A1C level is below 5.7 percent.
The fact sheet, called The A1C Test and Diabetes, offers in-depth information for people being tested. The A1C Test and Diabetes covers a wide range of information, including: how the test works; other blood tests for type 2 diabetes and prediabetes; accuracy of blood tests; where to learn more about A1C tests in people with hemoglobin variants; and A1C targets.Originally, the A1C test had been recommended only for monitoring diabetes. But in 2009, an international committee of experts convened by the American Diabetes Association, International Diabetes Federation and European Association for the Study of Diabetes recommended expanding the use of the test to include diagnosing type 2 diabetes and prediabetes. The test is convenient because it does not require fasting.Experts hope the ease of A1C testing will encourage more people to be checked for prediabetes and type 2 diabetes. Early identification and prompt treatment can delay or prevent type 2 diabetes and complications of the disease. The A1C test also helps providers adjust medication for people with diabetes to reduce the risk of long-term complications. About 26 million Americans are living with diabetes, and more than 7 million of them do not know it. Left untreated, diabetes can lead to heart disease, stroke, kidney disease, blindness, amputation, and other serious complications. An estimated 79 million adults have prediabetes, blood glucose levels that are higher than normal but not high enough to be called diabetes, which places people at increased risk for developing type 2 diabetes. Weight loss and increased physical activity or the drug metformin can delay or prevent type 2 diabetes, but fewer than 10 percent of people with prediabetes have been diagnosed.The standard blood glucose tests for diagnosing type 2 diabetes and prediabetes—the fasting plasma glucose test and the oral glucose tolerance test (OGTT)—measure blood glucose in a person who has not eaten anything for at least eight hours. The OGTT also measures blood glucose two hours after a person drinks a glucose-containing beverage. To confirm positive results, people should return on a different day to repeat the tests. The A1C test should also be repeated to confirm a diagnosis.If you are at least 45 years old, or younger than 45 and are overweight, inactive, and have at least one risk factor for type 2 diabetes, consider being tested for the disease. Risk factors include high blood pressure; high cholesterol; a family history of diabetes; a history of gestational diabetes; and African-American, Hispanic-American, Asian-American, Pacific Islander, or American Indian heritage.A1C results can be unreliable in some people. For example, people of African, Mediterranean or Southeast Asian descent, or people who have a family member with sickle cell anemia, may not know that they have a less common type of hemoglobin that can interfere with some A1C tests. If you have a family history of sickle cell or thalassemia, or A1C results seem very different from those of a blood glucose test, talk to your doctor about which A1C tests are appropriate for you.The fact sheet, The A1C Test and Diabetes, is available from NIDDK's National Diabetes Information Clearinghouse.
Maria Perno Goldie, RDH, MS
To read previous articles in RDH eVillage FOCUS written by Maria Perno Goldie, go to articles.