WRITTEN BY Margaret I. Scarlett, DMD based on an interview with Donna L. Mager, DDS, DMSc
If you think that salivary research is something that doesn’t relate to your practice, think again. There is an enormous amount of research in that area going on right now, and it will have implications for your practice. This is primarily due to advances in technology. The development of highly sensitive and specific diagnostic tests using “high throughput” systems will one day make the results of salivary tests rapidly available for use in your practice.
Saliva is already involved in the detection of HIV and human DNA testing, and its use will soon be expanded. In the future, certain systemic diseases will have specific salivary diagnostic techniques to identify or monitor them. Now, there is increasing evidence that saliva will be able to help diagnose disease, customize treatment, and monitor its success. Moreover, in dentistry, as well as medicine, certain people respond to care more or less successfully than others. Saliva is expected to unlock key information for tailoring treatment and improving overall health outcomes.
What do salivary researchers like Dr. Donna Mager look for? Her research examines the changes in oral soft-tissue microbiota that occur in diseases, such as insulin-dependent diabetes and oral cancer. Her findings suggest that when systemic diseases occur, the oral ecology favors shifts in bacterial colonization. The preliminary results of her studies suggest that disease conditions may have distinct microbial profiles, leading to early noninvasive diagnostic tests. If proven effective, salivary tests could monitor, and even suggest, treatment regimens.
In collaboration with the Dana-Farber Cancer Institute and Massachusetts General Hospital, it was found that elevated levels of three common oral bacteria indicated the presence of an oral cancer lesion with a sensitivity and specificity of over 80 percent and 82 percent respectively.
Dr. Mager says, “Our goal is to develop an early, noninvasive test that is highly diagnostic for oral cancer. Currently, we are improving the sensitivity and specificity of the salivary test and studying new populations. The second phase of the study will determine if our previous results are replicated. If so, we will conduct a prospective study of high-risk populations to determine if shifts in the microbiota can predict which individuals will develop dysplasias or precancers.”
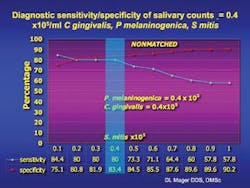
Diagnostic sensitivity and specificity of salivary counts x 105/ml C. gingivalis, P. melaninogenica, and S. mitis in the UNMATCHED group of 229 cancer-free and 45 oral cancer subjects. Percentage is shown on the Y axis, counts x105/ml of saliva are on the X axis. The blue line represents diagnostic sensitivity and the red line indicates specificity. The blue area highlights the sensitivity and specificity of salivary counts when C. gingivalis and P. melaninogenica were > 0.4x105/ml and counts of S. mitis reached 0.4x105/ml. Elevated salivary counts of 0.4 x105/ml in all 3 species resulted in a diagnostic sensitivity of 80% and specificity of 83.4% in the unmatched group.
Dr. David Wong at UCLA examines early indicators of carcinogenic changes in oral soft tissues. His team found that four types of messenger RNA from oral epithelial cells predicted the presence of an oral cancer in 90 percent of the cases studied. Still other investigations focus on cytokines (e.g., IL 1-beta or TNF-alpha, which modulate periodontal disease), COX-2, or other inflammatory mediators. The goal of these studies is to save lives by preventing, monitoring, and effectively treating oral cancer.
Currently, the five-year survival rate for oral squamous cell carcinoma is 54 percent. However, early detection improves these rates to 80 to 90 percent. Research is now focusing on early diagnostic techniques, since survival rates have not risen significantly in decades, despite dramatic improvements in oral cancer treatment. This is partly due to delays in detection and partly due to differences in the genetic make-up of oral squamous cell carcinoma. Investigators are examining genetic aberrations to establish which treatment regimens will be most effective for certain cancers.
Advances in microbiology have made salivary research an exciting frontier for dentistry. The old days of microbiology relied on culturing plates. (Remember blood agar and chocolate agar?) Salivary research now adds genetic procedures, such as Polymerase Chain Reaction (PCR), checkerboard DNA hybridization, or “reverse capture” hybridization, among others, to identify bacteria. These techniques are faster, more accurate, provide more information, and can identify uncultivable bacteria.
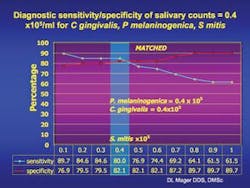
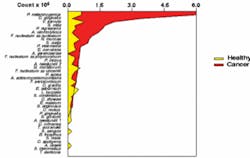
Mean DNA probe count of 40 common oral bacteria (x 105/ml) in saliva from 45 cancer-free and 45 oral cancer subjects matched by age, gender, and smoking status.
The checkerboard DNA hybridization technique involves denaturing bacterial DNA in oral samples by boiling. These separated strands of DNA are bound in parallel lanes to a membrane along with two standards. Pure DNA probes from 40 test species are given a fluorescent tag, denatured, and bound to the membrane in lanes at 90 degrees to the samples. When the DNA of a probe finds the complementary DNA strand in the sample, it binds to it and a fluorescent signal is emitted. Then, a fluorimager measures the pixel intensity of each signal for all test species in all samples. Voila! The numbers and proportions of bacteria are measured, and this information is stored in a computer.
You can get a whopping amount of information - 1,120 pieces of data on a single checkerboard. So, if eight or 12 membranes are processed at a time, with three to four sets completed in a week, a researcher like Dr. Mager can look at the levels of 40 species of bacteria from hundreds of people, in thousands of samples. This is in sharp contrast to the limited numbers of species, subjects, and samples that could be examined with traditional culture techniques. (Note: PCR and “reverse capture” are similar in that they measure bacterial RNA, instead of DNA, but they use different techniques.)
The salivary sleuthing continues. Dr. Mager is studying different subject populations to determine which bacteria are clinically important and which are not, and in what diseases they might be important for diagnosis or treatment. Dr. Mager is studying oral cancer and diabetes, while others at Forsyth are examining obesity, to see if these conditions impact the colonization patterns of the oral cavity. With an estimated 700 or more commensals (normal inhabitants), this becomes a formidable task. Luckily, the vast majority of these organisms live in harmony with humans - few bacteria are known to be pathogenic. In a state of health, oral pathogens are held in check by nonpathogenic species. In disease states, however, oral pathogens become destructive when they reach certain thresholds at particular sites. (Note: levels of 10 to the 7th power in many oral species are not unusual. Compare this to levels of 10 to the 11th power in colonic bacteria.) These facts, along with variations in host immune responses and environmental factors, impact oral health and complicate the many questions that these saliva super-sleuths are asking.
In order to study disease conditions, Dr. Mager began by examining the colonization of 229 healthy mouths. As there was no standardized method of sampling the oral soft tissues, she had to design a technique that could be reproduced for each person studied. It was expected that the microbiota of dental plaque would differ greatly from that of the oral soft tissue. Surprisingly, the microbial profiles of the soft tissues differed significantly from site to site. For example, the colonization patterns found on the tongue differed markedly from those of the buccal mucosa. Even tissues in close apposition, such as the ventral tongue and the floor of the mouth, differed significantly. Therefore, in healthy people, the site of the sample can be determined from the colonization patterns. The question then became: Would smoking or periodontal infections affect the microbial profiles in healthy subjects? The researchers found that, after adjusting for multiple comparisons, there were very few differences among the groups and these were not marked. When the study examined oral cancer subjects, however, dramatic differences were seen.
In oral cancer subjects, Dr. Mager found that when these three species of bacteria exceed a certain threshold, she can predict the presence of an oral cancer lesion with over 80 percent sensitivity and 82 percent specificity. While this is important, there is still a need for more studies to raise the sensitivity and specificity to over 90 percent for developing a saliva test. Researchers will conduct epidemiologic studies and examine more bacterial species to determine which combination of species might be most diagnostic. Fortunately, saliva is easily collected, but an international effort may be needed to acquire an oral cancer population large enough to conduct such studies. Dr. Mager hopes to develop a proof of concept for an early diagnostic test in the near future. She says, “We cannot, however, rule out the possibility that these species have a causal role in carcinogenesis.”
What theory may explain her results? The microbiota of saliva is quite similar to that of the mucous membranes. The soft tissues are continually shedding epithelial cells and the bacteria that colonize them into saliva. Bacteria must use powerful mechanisms of attachment, or “lock and key” mechanisms, to successfully colonize this shedding surface. Highly “species specific” adhesions attach to specific receptors on the surface of oral epithelial cells. If the oral epithelium becomes cancerous, it has been proven that cell membrane receptors are altered. This has been shown in vitro to change the species that are able to colonize oral cancer cell lines. Dr. Mager believes this change in colonization may exist in vivo and if so, would explain her results.
The best way to recognize the importance of saliva is to observe patients who don’t have any. Normal saliva is slightly alkaline, a pH of about 7.8. This feature, in addition to salivary buffers, helps to neutralize the acid produced by bacteria responsible for caries and regulate the oral pH. Patients with a history of radiation therapy are likely to have severe xerostomia if their salivary glands were in the path of the beam. Without daily fluoride treatments in custom-made trays, these patients are very likely to develop rampant decay. This occurs due to the loss of buffering and salivary minerals that help to remineralize early carious lesions. Extractions or infections that may result from decay can become life-threatening in oral cancer patients due to radiation damage, which damages oral blood vessels and impairs healing. Salivary substitutes like Laclede’s Biotene®, which have no alcohol in them, may provide transient relief. Most people rely on spray bottles of water, however, for continuous relief of severe xerostomia.
Due to the number of pharmaceutical agents and medical conditions that cause xerostomia, salivary flow is an important consideration for women dentists to include in their practice. Assessing salivary flow may be difficult, however. Many patients take multiple medications responsible for xerostomia - drugs that treat hypertension, allergies, insomnia, anxiety, and other disorders. Dr. Mager recommends daily use of neutral sodium fluoride, such as Colgate’s PreviDent®. For patients with severe xerostomia, such as that found in oral cancer subjects, daily neutral fluoride treatments in custom fluoride trays are a must.
Saliva will be more important in the near future for addressing the linkages between systemic and oral health, medications, and oral cancer. What does this mean? The clinician can be involved in research in a small way or moderate to big ways and still have a thriving practice. Clinicians can play a significant role in studies involving smoking, saliva, or other clinical issues. Such research offers opportunities for co-authorship in publications or teaching.
As a woman dentist, your role could include some teaching or research, as well as practice. To explore research, you can contact the lead investigator of a study that interests you. Many patients like knowing that they can make a difference in the lives of others by participating in studies as subjects or as controls - and they respect dentists for participating in academia or research. Getting involved offers new challenges that can be exciting for you and your patients ... and make a significant difference in the future of dentistry. ■
Margaret I. Scarlett, DMD
Dr. Scarlett is the science and women’s health editor for Woman Dentist Journal. An accomplished clinician, scientist, and lecturer, she is retired from the Centers for Disease Control and Prevention. You may contact her by email at [email protected].
Donna L. Mager, DDS, DMSc
Dr. Mager, an oral medicine specialist, is a researcher at the Forsyth Institute. She does her work in collaboration with the Dana-Farber Cancer Institute and Massachusetts General Hospital. She obtained her DDS degree from the University of Missouri and her DMSc from Harvard. Contact her at [email protected].