Introducing SomnoDent Herbst Advance and the SomnoDent Fusion
June 5, 2015
2 min read
The Aurum Group and Space Maintainers–Pacific Northwest have announced the latest FDA-approved products from SomnoMed, a developer of Continuous Open Airway Therapy (COATTM) products: SomnoDent Herbst Advance and SomnoDent Fusion. These two new options join the rest of the SomnoDent* product line: Flex, G2, Classic, Herbst, and Air.
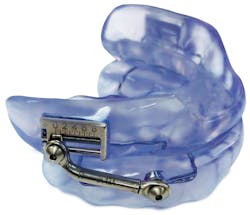
SomnoDent Herbst Advance Available in two models (Classic and Flex), the SomnoDent Herbst Advance is the first and only intraoral device featuring an easily viewable Plus (“+”) calibration indicator that shows the direction of advancement, giving clinicians and patients greater control over obstructive sleep apnea (OSA) therapy. Asymmetry in titration can be a thing of the past; the visual calibration indicator tells you exactly how far the device has been advanced from its start point. Developed with proprietary SMH BFlex, the SomnoDent Herbst Advance features a hinge mechanism that is more comfortable in the patient’s mouth, making it easier for the patient to remain compliant. With an 8-mm range of calibration and due to minimal necessary adjustment, chairtime is reduced and patients are ensured continuous therapeutic efficacy.SomnoDent Fusion
The new patented SomnoDent Fusion “fuses” wing- and screw-calibration technology together in one device, offering longer titration. The calibration flexibility offered in the SomnoDent Fusion means that it does not have to be reset as frequently, and less resets mean that patients won’t be without their devices during manufacturer adjustments. With exchangeable device wings that are closer to the occlusal surface, patients experience more room in their mouths and less bulk. Along with the flexibility in adjustment, the SomnoDent Fusion provides an 8.5-mm advancement—longer than most other dorsal-fin oral devices—as well as quick advancement with interchangeable wings and fine screw adjustments at .1-mm increments. The SomnoDent Fusion can also be made with proprietary SMH BFlex material to offer patients more comfort. Ultimately, the SomnoDent Fusion can save time and money with its drop-in fit, along with less device adjustments afterwards.
MORE READING:
Snoring or dying? There's a difference
Health history checklist for sleep-related breathing disorders
For more details, contact the Aurum Group/Space Maintainers–Pacific Northwest at (800) 423-6509.
*SomnoDent appliances are manufactured by Aurum Ceramic Dental Laboratories Co. under license from SomnoMed Limited and SomnoMed Inc.
About the Author
Sign up for our eNewsletters
Get the latest news and updates