Dental care providers depend on medical gloves to protect them and their patients against blood and other fluids that can spread infectious diseases such as AIDS and hepatitis B. Since many dental procedures involve direct contactwith blood and saliva, selecting the right gloves is a critical decision for dental professionals.
Natural rubber latex (NRL) has long been the preferred material for gloves, because it provides excellent barrier protection, comfort, and fit, and it is inexpensive. However, in recent years, the incidence of allergic reactions to latex proteins has raised new questions about glove choices.
The 2003 infection control guidelines published by the Centers for Disease Control and Prevention (CDC) now advise dental professionals to educate themselves about irritant and allergic contact dermatitis associated with glove use, including latex hypersensitivity. It is not surprising, then, that some are turning to synthetic substitutes out of health and safety concerns, even though many nonlatex gloves have inferior barrier protection and other properties.
However, a new generation of latex gloves has been developed that vastly reduces protein levels associated with latex sensitivity. Research shows that using these lower-proteingloves can decrease the incidence of latex reactions among health-care staff, thus making it possible for the majority of medical glove users to continue to rely on the proven barrier protection of latex gloves without heightened allergy concern.
Selecting gloves for optimal benefit
The single most important criterion in glove selection is barrier protection. Strength, fit, comfort, tactile sensitivity, ease of donning, and the ability to stretch, remain soft, conform to the hand, and enable the wearer to grip things are the other important requirements. Failure to provide any of these properties could compromise a glove’s barrier performance. It is, therefore, important that dental professionals understand the nature of each glove material and its ability to provide effective barrier protection.
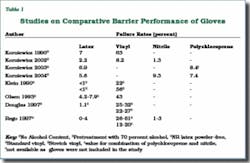
The commonly available medical gloves are made from NRL, vinyl, nitrile, and polychloroprene. Many studies have compared barrier performance of these gloves, particularly for NRL and vinyl. Natural rubber latex gloves have consistently demonstrated high levels of barrier protection (Table 1).
In addition, the natural rubber polymer gives latex gloves a unique resealing capability that makes them much less susceptible to viral penetration through tiny needle punctures. Nonlatex synthetic gloves cannot make this claim. For example, vinyl and nitrile gloves tend to tear easily once punctured and do not reseal.9,10
Natural rubber latex is considered to be the material of choice for glove barrier in the United States by the Food and Drug Administration (FDA Glove Powder Report, 1997). NRL gloves are regarded as the gold standard for barrier protection. Although the more expensive nitrile and polychloroprene gloves perform comparably, only latex combines barrier protection with the other important in-use characteristics of good fit, comfort, elasticity, tactile sensitivity, high tear resistance, and ease of donning. However, nitrile and polypropylene gloves are good candidates for latex allergic individuals who need to avoid latex proteins.
As for vinyl gloves, in view of their inferior barrier, they may be appropriate only for short-term tasks that involve minimum stress on the gloves and low risk of exposure to blood and other infectious materials. It is also worth noting that vinyl gloves containing highly toxic plasticizers, such as diethylhexylphthalate (DEHP), should not be used in contact with blood, fluids, or oily foods. The harmful effects of DEHP are well-documented.
Understanding latex sensitivity
There are three types of reactions associated with disposable medical gloves:
➊ Irritant contact dermatitis
➋ Type IV allergy of delayed hypersensitivity
➌ Type I allergy of immediate hypersensitivity
Only one of the three - Type I allergy of immediate hypersensitivity - is a reaction to the residual latex proteins in gloves. The other two types are reactions due to irritants and some residual chemicals employed during the manufacture and processing of medical gloves. They can occur with both latex and synthetic gloves, and are potentially less serious.
While the occurrence of both irritant contact dermatitis and delayed hypersensitivity is more rampant, studies show that only about 1 percent of the general population is affected by Type I allergy - the latex protein allergy. The prevalence among health-care workers, however, is higher (3 to 16 percent) due to repeated exposure to excessive residual latex proteins.11
It is noteworthy that Type I allergy is not caused only by latex proteins. It can also be caused by many other proteins such as those found in some foods - bananas, watermelon, kiwi, potatoes, tomatoes, peanuts, fish, meat, and eggs. Proper diagnosis (through a skin prick test administered by a medical doctor) is important to identify the relatively small group of individuals who are hypersensitive to latex proteins.
Once diagnosed, allergic individuals have to avoid the allergens they are sensitive to, while the nonaffected majority, as in the case of glove use, needs to minimize their excessive exposure to potential allergens.
Product improvement
Latex gloves are made from the milky fluid from the trees of Hevea brasiliensis. Like all plant materials, Hevea latex contains proteins (about 1 percent), as well as rubber (about 30 percent, in the form of particles) among other substances. The soluble proteins present in the latex serum phase are largely lost when latex is processed into gloves, often leaving behind only a small amount of residual extractable protein. Not all of these residual extractable proteins cause allergic reactions.
Because they are water soluble, many of these remaining proteins can be removed from latex gloves by proper processing. Improved manufacturing technologies can now effectively reduce this residual extractable protein fraction via appropriate pre- and post-leaching protocols and the chlorination process.12
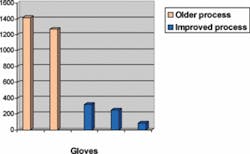
These new manufacturing techniques are far superior to those that were in use at the dawn of the AIDS epidemic, when the demand for latex gloves spiked and hurriedly manufactured gloves with high levels of protein began to flood the market. This older generation of gloves contained levels as high as 1,000 to 2,000 micrograms of residual extractable protein per gram of glove - high enough to cause sensitization and trigger allergic reactions in latex-sensitive individuals.12 By contrast, the current generation of latex gloves using these new techniques can have levels as low as 100 micrograms and less, especially for powder-free latex gloves (as estimated by the Modified Lowry Test). Aeroallergenicity, or the potential of becoming aeroallergens, is also greatly reduced.
Table 2, which compares five brands of latex gloves,13 demonstrates that powdered latex gloves produced using the older manufacturing technology contain significantly higher protein content (1P and 2P) than the low-protein gloves (powdered and powder-free) manufactured by improved processes (3P, 4P, and 5PF).
Reducing incidence of latex sensitization
It has long been suspected that using latex gloves with reduced amounts of latex proteins would lower the number of latex-related incidences among health-care staff. Recent hospital studies14-20 in the United States, Canada, and Europe confirm that wearing low-protein, low- or nonpowdered latex gloves greatly diminishes the risk of allergic reactions and the likelihood of health-care workers developing latex sensitivity.
Studies also have shown that the use of low-protein powdered or nonpowdered gloves allowed latex-sensitive individuals donning synthetic gloves to work safely alongside colleagues wearing low-protein latex gloves. One of the studies16 found that even latex-allergic individuals could use natural rubber latex gloves with very low allergenic protein content, although it is generally recommended that such individuals continue to wear synthetic substitutes.
The American Dental Association’s own research confirms this trend. In 1994, the ADA began investigating the prevalence of latex allergy among dentists, dental hygienists, and dental assistants, and found that 6.2 percent of workers tested positive for Type I latex hypersensitivity.21 Data from the subsequent five years indicated a decline in prevalence from 8.5 percent to 4.3 percent.22 The Centers for Disease Control and Prevention suggested this downward trend as being due “to use of latex gloves with lower allergen content.” (Guidelines for Infection Control in Dental Health-Care Settings, December 200323)
Meeting the challenge
To address the latex allergy problem, Malaysia, the largest supplier of medical gloves (latex and synthetic) to the United States, has formulated the Standard Malaysian Glove (SMG), a certification program in consultation with the U.S. Food and Drug Administration which ensures the manufacture of high-quality, low-protein latex with high barrier performance and low risk to latex protein allergy. SMG-certified latex gloves must consistently meet stringent barrier and tensile specifications as well as rigorous standards for protein and powder content. These gloves are now available in powder-free and lightly powdered varieties.
ECRI, a nonprofit health research company and the world’s largest independent evaluator of biomedical equipment, also recommends the use of low-protein latex gloves that have protein levels printed on their labeling or that are SMG-certified, particularly the powder-free variety, because these gloves are guaranteed to have low protein and powder limits. (Health Devices, May 200426)
As a matter of precaution, latex-sensitive individuals should avoid using latex gloves and select nonlatex or synthetic gloves that provide adequate barrier protection.
Final words
Because dentists, dental hygienists, and dental assistants frequently come in direct contact with saliva and blood, the donning of suitable medical gloves for effective barrier protection is of great importance. Deciding which type of glove to use for which patient and procedure can be a tough choice, especially after weighing the health and safety concerns of various glove types.
With the advent of lower-protein latex gloves like SMG, the majority of dental professionals who are not latex-sensitive can continue to use latex gloves and take advantage of latex’s excellent barrier performance properties without endangering their latex-sensitive co-workers. The use of this new generation of gloves, especially the powder-free variety, can also serve to vastly reduce the risk of developing sensitivity to latex.
For more information about the SMG programs, visit http://www.smg-online.biz/.
References
1 Korniewicz DM, Laughon BE, Cyr WH, Lytle CD, Larson E. Leakage of virus through used vinyl and latex examination gloves. Journal of Clinical Microbiology 1990; 28:787-788.
2 Korniewicz DM, El-Masri M, Broyles JM, Martin CM, O’Connell KP. Performance of latex and nonlatex medical examination gloves during simulated use. Am J Infection Control 2002; 30:133-137.
3 Korniewicz DM, Garzon L, Plitcha S. Health-care workers: risk factors for nonlatex and latex gloves during surgery. J Am Ind Hyg Assoc 2003; 64(6):851-855.
4 Korniewicz DM, Garzon L, Seltzer J, Feinleib M. Failure rates in nonlatex surgical gloves. Am J Infection Control 2004; 32:268-275.
5 Klein RC, Party E, Gershey EL. Virus penetration of examination gloves. Biotechniques 1990; 9:196-199.
6 Olsen RJ, Lynch P, Coyle MB, Cummings J, Bokete T, Stamm WE. Examination gloves as barriers to hand contamination in clinical practice. JAMA 1993; 270:350-353.
7 Douglas A, Simon T, Goddard M. Barrier durability of latex and vinyl medical gloves in clinical settings. Am Ind Hyg Assoc J 1997; 58:672-6.
8 Rego A, Roley L. In-use barrier integrity of gloves: latex and nitrile superior to vinyl. American Journal of Infection Control Oct. 1999; 27(5):405-410.
9 Hasma H, Othman AB. Barrier performance of NR, vinyl, and nitrile gloves on puncture. Paper presented at the Latex 2001 Conference, RAPRA, Munich, Germany.
10 Broyles JM, O’Connell KP, Korniewicz DM. A PCR-based method for detecting viral penetration of medical exam gloves. J Clin Microbiol 2002; 52:965-999.
11 Liss GM, Sussman GL. Latex sensitization: occupational vs. general population prevalence rates. American Journal of Industrial Medicine 1999; 35:196-200.
12 Yip E, Cacioli P. The manufacture of gloves from natural rubber latex. J Allergy Clin Immunology 2002; 110:S3-14.
13 Yeang HY, et al. Studies on latex gloves and proteins. Rubber Research Institute of Malaysia 2003.
14 Allmers H, Brehler R, Chen Z, Raulf-Heimsoth M, Fels H, Baur X. Reduction of latex aeroallergens and latex-specific IgE antibodies in sensitized workers after removal of powdered natural rubber latex gloves in a hospital. J Allergy Clin Immunol 1998; 102:841-46.
15 Tarlo SM, Easty A, Dubanks K, Min F, Liss G. Outcomes of a natural rubber latex control program in an Ontario teaching hospital. J Allergy Clin Immunol 2001; 108:628-633.
16 Turjanmaa K, Kanto M, Kautiainen H, Reunala T, Palosuo T. Long-term outcome of 160 adult patients with natural rubber latex allergy. J Allergy Clin Immunol 2002; 110:S 70-74.
17 Hunt LW, Kalker P, Reed CE, Yunginger JW. Management of occupational allergy to natural rubber latex in a medical center: the importance of quantitative latex allergen measurement and objective follow-up. J Allergy Clin Immunol 2002; 110:S94-106.
18 Allmers H, Schmengler J, Skudlik C. Primary prevention of natural rubber latex allergy in the German health-care system through education and intervention. J Allergy Clin Immunol 2002; 110(2):318-323.
19 Kelly KJ, Klancnik M, Kurup V, Barrios-Jankol C, Fink JN, Petsonk EL. A four-year prospective study to evaluate the efficacy of glove interventions in preventing natural rubber latex sensitization in health-care workers at two hospitals. J Allergy Clin Immunol 2003; Part 2: 111(2), No. 426.
20 Rueff F, Schopf P, Przybilla B. Parameters of natural rubber latex (NRL) sensitization decrease in health-care workers (HCW) following reduction of NRL exposure. Klinik und Poliklinik fur Dermatologie und Allergologie, Ludwig-Maximilians-Universitat, Munich, Germany. Presented at the 56th annual meeting of the American Academy of Asthma, Allergy, and Immunology (AAAAI) in 2000.
21 Hamann CP, Turjanmaa K, Rietschel R, et al. Natural rubber latex hypersensitivity: incidence and prevalence of type I allergy in the dental professional. J Am Dent Assoc 1998; 129:43-54.
22 Siew C, Hamann C, Gruniger SE, Rodgers P, Sullivan KM. Type I latex allergic reactions among dental professionals, 1996-2001. Journal of Dental Research 2003; 82 (Special Issue):1718.
23 CDC Recommendations and Reports: Guidelines for Infection Control in Dental Health-Care Settings, Centers for Disease Control and Prevention, Dec. 19, 2003.
24 NIOSH Alert: Preventing allergic reactions to natural rubber latex in the workplace. Centers for Disease Control and Prevention, National Institute of Occupational Safety and Health 1997.
25 Technical Information Bulletin: Potential for allergy to natural rubber latex gloves and other natural rubber products, U.S. Department of Labor, Occupational Safety and Health Administration 1999.
26 ECRI Guidance Article. Lower-protein gloves: a way to reduce allergic reactions in hospital staff. Health Devices May 2004; 33(5):169-174. ■
Esah S. Yip, DSc
Dr. Yip has been engaged in research on latex and rubber properties for more than 30 years. She is currently the director of the Malaysian Rubber Export Promotion Council, and is charged with informing glove users of up-to-date developments in medical gloves. Contact her at [email protected].