Warning against calcitonin salmon for osteoporosis
Osteoporosis is a systemic skeletal disease characterized by low bone mass and structural deterioration of bone tissue leading to bone fragility and increased risk of fracture. Based on NHANES III data, it is estimated that approximately 10 million people in the U.S. have osteoporosis and another 34 million have low bone mass (osteopenia).(5) The goal of therapy is to reduce the risk of fracture.
For postmenopausal osteoporosis, there are currently two approved indications: 1. Treatment of osteoporosis in postmenopausal women (or women at high risk of fracture, as with Forteo and Prolia); 2. Prevention of osteoporosis in postmenopausal women.Osteoporosis is mainly diagnosed using bone mineral density (BMD) techniques based on the diagnostic criteria set forth by the World Health Organization (WHO) in 1994.(6) However, experts agree that BMD alone is not sufficient to accurately predict fracture risk. This led to the development of a new risk assessment tool for prediction of osteoporotic fracture (FRAX).(7)
The FRAX algorithms, developed by the WHO in 2008, include clinical risk factors that predict an increased risk of fracture (age, sex, prior fragility fracture after age 50 years, history of corticosteroid use [the equivalent of ≥ 5 mg of prednisone for more than three months], parental history of hip fracture, rheumatoid arthritis, secondary osteoporosis, current tobacco use, alcohol use of greater than 2 units daily, and low body mass index). Using the FRAX tool, fracture risk is reported as the 10 year risk of hip fracture and the 10-year risk of major osteoporotic fracture.
Currently, the National Osteoporosis Foundation recommends treatment be considered for patients who have had an osteoporotic fracture, patients with a BMD T-score of <-2.5 (2.5 standard deviations (SD) below the young adult mean), and patients over age 50 years with low bone mass (T-score -1.0 to -2.5) with 10-year risk probability of >3% for hip fracture or >20% for major osteoporotic fracture as obtained using the FRAX algorithm.(8)
To view a PDF of the WHO Fracture Risk Assessment Tool(9), click here.
The FDA will make a final determination after considering the advisory committee's recommendations.
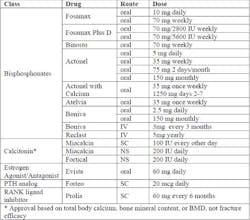
Approved Products for the Treatment of Post-Menopausal Osteoporosis(2)
(Reporting by Toni Clarke in Washington; Editing by Leslie Adler)
http://news.yahoo.com/fda-panel-advises-calcitonin-salmon-not-used-osteoporosis-221820123--finance.html