Lasers and bacterial reduction: A comprehensive technique to treat chronic periodontitis
Use the laser light to eliminate periodontal pathogens and promote the return of the periodontium to a state of health.
Throughout the last decades, it has been well-recognized and understood that most periodontal diseases are infections caused by microorganisms, especially bacteria. These microorganisms (pathogens) colonize the tooth surface at or below the gingival margin and are organized in entities called biofilms.
According to Socransky and Haffajee's1 review of several publications discussing the effect of biofilm in humans, the onset of these diseases is usually delayed for prolonged periods of time after initial colonization by the pathogen(s), and the course of these diseases typically can run for years. The causative agents in most instances appear to be members of the indigenous microbiota and, thus, the infections can be considered endogenous.1
The classical protocol used to treat such periodontal infections is scaling and root planing. Sometimes, however, pathogenic subgingival bacteria are difficult to eliminate, and despite repeated treatment sessions, they grow back and repopulate the periodontal pocket. These microorganisms usually belong to specific groups such as Porphyromonas gingivalis (Pg), Bacteroides forsythus (Bf), and Treponema denticola (Td), which are considered strongly related to periodontal diseases. Clinically, the result of this chronic infection is the progressive destruction of the periodontium and tooth loss. In addition to the bacterial flora, inadequate oral hygiene habits allowing accumulation of plaque on the tooth surface, the genetic component of the individual, the use of tobacco (smokers), and some systemic diseases all are considered risk factors for periodontal disease and can have a detrimental effect on the treatment outcome or change the course of the disease for the worse.
The source of the infecting pathogens for any given individual is usually unknown, although transfer from parents or persons of physical contact is thought to play a primary role. The treatment of these infections is complex and usually consists of physical, antimicrobial, and ecological approaches.1
In this article, we will discuss the use of laser light as a new modality of anti-infective approach to treat biofilm/plaque-induced periodontal diseases. The laser light is used in this context as a complement to the routine techniques of treating periodontal diseases, with the objective of eliminating periodontal pathogens through its antibacterial property and promoting the return of the periodontium to a state of health through its anti-inflammatory effect.
The laser light application as an adjunct to soft-tissue management in dentistry has seen an improvement in recent years.
A laser is a photo-thermal device that produces a monochromatic, coherent, and collimated light with a specific wavelength. It does not have the specific lock-and-key chemical target. It acts directly on cellular structures, destroying cell walls, altering DNA, modifying metabolic processes, and ungluing the polysaccharide structure of the biofilm.2 Some have reported reduction of subgingival bacterial flora in vitro. 3 Others have examined the in vivo bactericidal effect of certain lasers, which could significantly reduce the levels of some subgingival periodontal pathogens.4,5 In contrast, still other reports have questioned the benefit of subgingival laser therapy compared to that of conventional scaling and root planing.6
Many publications have shown investigations in basic, applied, and clinical sciences of the laser. Utilizing histological and thermal assessment techniques, applied science explores ranges of use, defines safe operating parameters for specific use, and discovers when adverse events might be anticipated, providing an understanding of the laser/tissue interaction during a specific treatment.7
Generally, lasers are identified by an active medium and receive the name of the generating crystal — for example, Nd:YAG (Neodymium: Yttrium-Aluminum-Garnet), Er:YAG (Erbium: Yttrium-Aluminum-Garnet), Diode (Gallium, Arsenide Aluminum), etc. Each has a set range of parameters and specifications. Emission wavelength, power, repetition rate, energy per pulse, energy density, and delivery all affect interaction with dental soft tissue. The specific combination of parameters is what determines the context for well-controlled applied scientific investigation of current and future use.8
In other words, each laser apparatus and its set wavelength have its specific indication of use according to the desired treatment outcome. Therefore, the operator must select the laser apparatus according to the procedure that will be performed and the target tissue involved. The operator must also know what parameters to use based on previous studies that have set the guidelines for that particular procedure.8
With that in mind, we will present some periodontal treatment outcomes in which conventional periodontal techniques were combined with the laser light to control chronic periodontitis.
In our pool of patients, we mostly have those who have been treated conventionally for periodontal disease and either have not responded and continue to break down, or who have responded poorly to conventional periodontal treatment and need frequent maintenance recalls. Despite all treatment and compliance efforts, these patients continue to see a worsening of their periodontal condition.
These patients were characterized by the type of periodontal disease that they presented with at the time of diagnosis and by the bacterial flora identified in the periodontal pockets. Through the use of microbiological culture and/or DNA probes, the periodontal pockets were sampled and the harvested microorganisms were characterized.
The bacterial flora infecting this patient population usually included pathogenic strains from the Actinobacillus actinomycetemcomitans (Aa) group and/or Porphyromonas gingivalis (Pg), Prevotella intermedia (Pi), Bacteroides forsythus (Bf), Treponema denticola (Td) group and other less pathogenic bacteria. This finding is significant because it identifies a difficult-to-treat periodontal infection that can see periods of remission and exacerbation, setting the chronic stage for an infection that cannot be easily arrested by conventional periodontal therapy, thus causing a continuous breakdown of the periodontal structures and early tooth loss.9
In such cases, after identifying the pathogenic microflora, we were able to use specific anti-infective therapy (confirmed by antibiogram in cases where antibiotics were indicated) in addition to conventional scaling and root planing. Chemotherapeutic substances can be used either through local delivery directly in the periodontal pocket or systemically through oral use of antibiotics. In either case, we know that there must be a "lock-and-key" situation where the chemical has a potential action over the microorganism present in the periodontal pocket. Otherwise, the infecting pathogens can survive the therapy and perpetuate the disease. On the other hand, when using a large-spectrum therapy without specificity (like the laser light), the chances of eliminating microorganisms are greater.
Clinical case
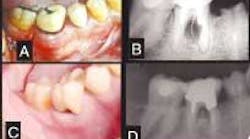
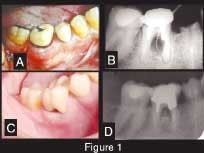
A 42-year-old Caucasian male presented to our clinic for evaluation of a mandibular molar. His previous dentist wanted to extract the tooth, but the patient wanted a second opinion. The patient stated that he would do anything possible to save the tooth. His overall health was good, he did not smoke, he had excellent oral hygiene, and he exhibited no signs of generalized periodontal disease. A year before this initial contact, the patient underwent dental treatment of Tooth No. 30 to repair an ill-fitting post and a crown. He related that some type of surgery was done in the area of the buccal furcation of this tooth to remove part of the post that had apparently perforated the root. After this treatment, he noticed a swelling on the buccal aspect of the furcation that had periods of exacerbation and remission. Despite several attempts to scale and cleanse the area, the problem could not be resolved. The dentist recommended extraction. A radiograph showed a radiolucent lesion in the furcation with a diameter of approximately 0.5 cm (Figure 1). We made another attempt to convince the patient to have the tooth removed and an implant placed, but we were unsuccessful.
Figure 1 — Clinical and radiographic aspect. A: Initial presentation. Observe swelling and redness at the buccal aspect of Tooth No. 30. B: Periapical radiograph showing the radiolucency at the furcation area (pocket depth of 9 mm). C: Clinical presentation four years later. Observe the absence of exudate, the gingival opening at the furcation allowing the patient to clean the pocket, and the porcelain crown in place. D: Periapical radiograph showing the furcation area four years later (pocket depth of 5 mm).
Therefore, the alternative treatment for the area consisted of scaling and placement of an Actisite® fiber (tetracycline) that remained in the pocket for seven days. Upon removal a week later, a flap surgery was performed, the area was again cleansed, and a bone graft of Perioglass® and a membrane of Capset® were placed. Three months later, a new infectious exudate was observed in the furcation area. All pockets around Tooth No. 30 were 1 to 2 mm deep except the distal aspect of the mesial root, which was 9 mm deep. A DNA probe of this particular area showed an extremely high total bacterial load consisting of 32 percent periodontal pathogens. Pg accounted for approximately 20 percent of the pathogens (Figure 2-A). The local-delivery antibiotic and the periodontal surgery with placement of bone graft all failed to kill the bacteria in that pocket and restore the area to health.
At this point we opted to use the laser light with the intent of reducing pocket pathogens and to try to seal the perforated root via furcation. Several studies have shown that certain lasers have the ability to promote a "melting" of dental mineralized tissues and seal dentinal tubules. The laser of choice was the Nd:YAG due to its property of bacterial reduction and sealing of cementum. The protocol used was 1.5 watts, 15 hertz, 15 seconds in the furcation parallel and along the distal aspect of the mesial root of Tooth No. 30, and another 15 seconds perpendicular to the root. This procedure was repeated after seven days and a third application was performed 30 days after the first one.
The post was not removed because of the risk of fracturing the roots. A new preparation was performed, a temporary crown was placed, and the tooth was put back in occlusion. No clinical signs or symptoms of periodontal infection were noticed.
Six months after the initial DNA probe, another sample was harvested from the same area previously treated with laser light. The results showed a dramatic decrease in periodontal pathogens. In addition, the pocket depth had been reduced from 9 mm to 5 mm. The total bacterial load went from 22 million bacteria to approximately 6 million, only 2 percent of which were periodontal pathogens instead of the initial 32 percent. The counts for Pg specifically went from approximately 20 percent to .05 percent, with no treatment for four months (Figure 2-B).
Figure 2 — Bacterial DNA probe results. A: initial test showing the presence of a high Total Bacterial Load (TBL) = 22.86 million and a high percentage (TML = 32 percent) of periodontal pathogens called total marker load (Pg = 19.8 percent, Bf = 12.2 percent). B: Bacterial DNA probe six months later showing both the reduction of the TBL (5.95 million) and the reduction of periodontal pocket pathogens (Pg = .5 percent, Bf = 1.7 percent) The total marker load (TML) percentage went down to 2 percent. C: Eighteen months later, the bacterial DNA probe result shows a TBL of 7.76 million bacteria and an absolute absence of periodontal pathogens, suggesting that the periodontal pocket was repopulated by normal flora only and has been maintained like that throughout time in agreement with the clinical aspect of the referred tooth.
At this time, another single laser application was performed using the same parameters as before. The patient only returned to the clinic a year later. No treatment was performed during this time, and the patient was asymptomatic. To monitor the bacterial flora of the pocket, a sample of subgingival plaque was taken and sent for DNA analysis. The total bacterial load had increased to 7.76 million bacteria, but none of them were pathogenic, meaning that the sulcus had been repopulated by nonpathogenic bacteria (Figure 2-C). The laser light treatment was able to eliminate pathogens from the periodontal pocket and create an environment in which only normal flora returns and survives. This effect was observed during an entire year during which no periodontal treatment was rendered, according to the patient's wishes. The laser light treatment also shows a long-lasting effect on the shift of bacterial subgingival flora. The source of the infection — the ill-fitting post that was not replaced — was probably isolated from the periodontal environment due to the sealing of mineralized tissues.8
At this point, the patient was referred to a prosthodontist, who placed a porcelain crown on Tooth No. 30. The patient remains asymptomatic today. The periodontal pocket on the distal aspect of the mesial root remains 5 mm deep; bacterial flora is being maintained at the indigenous level; and Tooth No. 30 is totally functional.
Several other patients with chronic periodontitis have been treated in our clinic following this same protocol:
- Review of the patient's medical history, medications, and presence of possible risk factors for periodontal disease
- Periodontal charting and confirmation of the periodontal diagnosis
- Identification of the pocket microflora using microbiological culture or DNA probe, and antibiogram with antibiotic sensitivity test
- Scaling and root planing and oral hygiene instruction
- Laser bacterial reduction
- Maintenance therapy
Currently, a formal clinical pilot trial is underway to confirm our clinical results in the private practice arena. We firmly believe that there are certain types of periodontal disease that cannot be controlled by conventional therapy. Therefore, the addition of the laser light to the practice of periodontology will give the experienced periodontist a new and efficacious alternative to treat chronic periodontitis.
References
- Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontology 2000 2002; 28:12-55.
- Cortes M. The elimination of bacteria and biofilms in periodontal disease via the thermal laser. International Congress Series 2003; 1248: 359-362.
- White J, Gekelman D, Budd J. Lasers and dental soft tissues: reflections on our years of research. International Congress Series 2003; 1248:13-19.
- Cobb CM, McCawley TK, Killoy WJ. A preliminary study on the effects of the Nd:YAG laser on root surfaces and subgingival microflora in vivo. J Periodontol 1992; 63:701-707.
- Ando Y, Aoki A, Watanabe H, Ishikawa I. Bactericidal effects of erbium YAG laser on periodontal bacteria. Lasers Surg Med 1996; 19:190-200.
- Radvar M, Creanor SL, Gilmour WH, et al. An evaluation of the effects of an Nd:YAG laser on subgingival calculus, dentin, and cementum: An in vitro study. J Clin Periodontol 1995; 22:71-77.
- White JM, Goodis HE, Setcos JC, Eakle S, Hulscher BE, Rose CL. Effects of pulsed Nd:YAG laser energy on human teeth: a three-year follow-up study. J Am Dent Assoc 1993; 124:45-51.
- Eduardo CP, Eduardo FP, Haypek P. Restorative dentistry and esthetics with lasers. International Congress Series 2003; 1248:91-99.
- Baehni P. Microbiological diagnosis in periodontics. Schweiz Monatssch Zahnmed 1995; 105:798-808.
Erica Krohn Jany Migliorati, DDS
Dr. Migliorati graduated from the University of Sao Paulo, Brazil, and is a periodontist. She currently is an assistant professor in the Department of Periodontology at the Nova Southeastern University College of Dental Medicine. Contact Dr. Migliorati at [email protected].