Columbia Group points to genetic signature for classifying periodontal disease
Researchers say new system may allow
for earlier diagnosis and personalized treatment of severe periodontitis
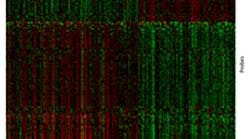
By looking at the expression of thousands of genes in gum tissue, researchers can now classify most cases of periodontitis into one of two clusters. More severe cases of the disease are represented under the red bar, less severe cases under the blue bar. The findings may allow for earlier diagnosis and more personalized treatment of severe gum disease, before irreversible bone loss has occurred.Image credit: Panos N. Papapanou, DDS, PhD/Columbia University College of Dental Medicine
Researchers at Columbia University Medical Center (CUMC) have devised a new system for classifying periodontal disease. The findings were published recently in the online edition of the Journal of Dental Research.
The system is based on the genetic signature of affected tissue, rather than on clinical signs and symptoms. The new classification system may allow for earlier detection and more individualized treatment of severe periodontitis — before loss of teeth and supportive bone occurs.
Currently, periodontal disease is classified as either “chronic” or “aggressive,” based on clinical signs and symptoms, such as severity of gum swelling and extent of bone loss.
The study leader, Dr. Panos N. Papapanou, professor and chair of oral and diagnostic sciences at CUMC’s College of Dental Medicine said, “However, there is much overlap between the two classes. Many patients with severe symptoms can be effectively treated, while others with seemingly less severe infection may continue to lose support around their teeth even after therapy. Basically, we don’t know whether a periodontal infection is truly aggressive until severe, irreversible damage has occurred.”
Looking for a better way to classify periodontitis, Dr. Papapanou turned to cancer as a model. In recent years, cancer biologists have found that, in some cancers, clues to a tumor’s aggressiveness and responsiveness to treatment can be found in its genetic signature. To determine if similar patterns could be found in periodontal disease, the CUMC team performed genome-wide expression analyses of diseased gingival (gum) tissue taken from 120 patients with either chronic or aggressive periodontitis. The test group included both males and females ranging in age from 11 to 76 years.
The researchers found that, based on their gene expression signatures, the patients fell into two distinct clusters.
“The clusters did not align with the currently accepted periodontitis classification,” said Dr. Papapanou.
However, the two clusters did differ with respect to the extent and severity of periodontitis, with significantly more serious disease in Cluster 2. The study also found higher levels of infection by known oral pathogens, as well as a higher percentage of males, in Cluster 2 than in Cluster 1, in keeping with the well-established observation that severe periodontitis is more common in men than in women.
“Our data suggest that molecular profiling of gingival tissues can indeed form the basis for the development of an alternative, pathobiology-based classification of periodontitis that correlates well with the clinical presentation of the disease,” said Dr. Papapanou.
The researchers’ next goal is to conduct a prospective study to validate the new classification system’s ability to predict disease outcome. The team also hopes to find simple surrogate biomarkers for the two clusters, as it would be impractical to perform genome-wide testing on every patient.
The new system could offer huge advantages for classifying people with different types of periodontitis.
“If a patient is found to be highly susceptible to severe periodontitis, we would be justified in using aggressive therapies, even though that person may have subclinical disease,” said Dr. Papapanou. “Now, we wait years to make this determination, and by then, significant damage to the tooth-supporting structures may have occurred.”
The paper is titled, “Gingival Tissue Transcriptomes Identify Distinct Periodontitis Phenotypes.” The other contributors are M. Kebschull (CUMC), R.T. Demmer (CUMC), B. Grün (University of Linz, Linz, Austria), P. Guarnieri (CUMC), and P. Pavlidis (University of British Columbia, Vancouver, BC, Canada).
The authors declared no financial or other conflicts of interests. The study was supported by grants from the National Institute of Dental and Craniofacial Research (DE-015649 and DE-021820), the National Center for Advancing Translational Sciences (UL1-TR000040), the National Institutes of Health (R00 DE-018739), Colgate-Palmolive, the German Research Foundation (KFO208, TP6 and TP9), the German Society for Periodontology, the German Society for Oral and Maxillofacial Sciences, the Neue Gruppe, BG by the Austrian Science Fund (V170-N18), and the Michael Smith Foundation for Health Research.