Visiting the dentist can be very unpleasant for up to 20% of dental implant patients. In these cases, an invasive surgical procedure called a sinus lift is required. This involves separating the delicate membrane from the floor of the maxillary sinus above the upper jaw in order to insert a bone graft and make the jawbone thick enough to support an implant.
RELATED |The use of stem cells in dental implant site development
RELATED |Saving teeth through surgical endodontics
Sinus lifts can be a particularly unpleasant experience for patients. The procedure takes more than an hour and involves significant trauma. Patients need to rest at home for three to 10 days due to the pain, discomfort, and severe, unattractive facial swelling and bruising. Six to nine months after the procedure, new bone is generated, and a second procedure is required to insert a dental implant into the newly formed bone. This is followed by a three- to five-month waiting period for the dental implant to be integrated into the bone when the prosthetic tooth can finally be inserted.
A new dental implant called the iRaise™ Sinus Lift Implant, developed by Israeli-based Maxillent Ltd., provides a solution that is far less traumatic and painful, minimizes swelling, and allows patients to return to their normal lives a day or less after the procedure. The system involves a closed sinus lift implant that increases the bone’s thickness and provides a dental implant in one minimally invasive procedure. Instead of reaching the sinus and placing a bone graft through an aggressive surgical approach, the iRaise implant uses a hollow channel to allow fluid to be injected through the implant to lift the sinus membrane.
Click here to watch a video to see how iRaise works.
As background, the maxillary sinuses are two hollow cavities located behind the cheekbones and just above the upper molar teeth in the back of the mouth. As people age, the sinuses expand and the jawbone shrinks in size. If upper molars are lost, the bone reduction is even more severe. In more than 50% of these cases, the remaining jawbone lacks sufficient volume to support a dental implant.
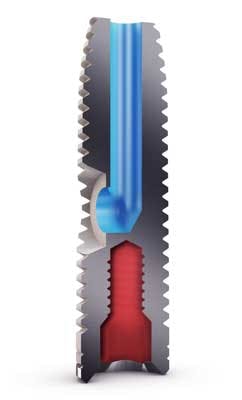
The iRaise procedure takes 20 to 25 minutes under local anesthesia, compared to the standard hour-long procedure that often uses stronger intravenous sedation. After drilling safely into the sinus floor, without damaging the delicate sinus membrane, the iRaise implant is partially inserted, whereby the tip reaches the sinus but the side opening of the implant, connected to a disposable tube connector, remains outside the body. The tube connector hooks onto the implant and allows fluid to be injected through the iRaise implant. Using a syringe, two to three cc of saline are injected underneath the Schneiderian sinus membrane to separate and elevate it from the sinus floor. The saline is then drained out with the syringe, which allows the membrane to descend and rest on the membrane floor without any adhesion.iRaise implant with tube connector
In the same amount as the injected saline solution, bone graft material, in a consistency comparable to toothpaste, is then injected into the same volume to build upon the existing bone of the sinus floor. The bone’s thickness can be increased, starting from 4 mm to 8 mm, to the height of the implant, which is typically 13 mm to 16 mm. The tube connector and syringe are removed, and the implant is fully inserted into the sinus floor. With the implant fully inserted into the bone, the channel’s openings, whereby fluids enter from the side of the implant and exit from the top, are sealed within the bone, cutting off any opening for bacteria from the mouth. Six to nine months later, healthy, native bone in the full volume of the bone graft is formed for a firm structural support for the implant. To date, all patients with iRaise have been able to return to full activity the same day or the day after, and experience minimal bruising or swelling.
With iRaise, a surgical procedure that requires surgical training is transformed into an implant procedure that can be performed by maxillofacial surgeons, periodontists, and dentists with dental implant experience. There is a minimal learning curve for experienced dentists because they are using the same kinds of tools and skills as with dental implants.
The addressable global market for iRaise is about $1.5 billion yearly, representing about one million sinus lifts for dental implants, mostly for upper molars. The CE mark for iRaise was granted in April 2011, with 510(k) de-novo approval likely in early 2014. Maxillent has raised $6 million to date and is now engaged in a $4 million to $5 million Series A round which should close by early 2013. Major dental implant players are carefully watching Maxillent’s development, but there have yet to be any active discussions about partnering. Meanwhile, the company is expanding its technology to save surgical time and trauma in the dental implant market.
iRaise began selling earlier this year in its home market of Israel and has since expanded to France, Hong Kong, Italy, and other European countries. U.S. sales will be handled by distributors. The mostly patient out-of-pocket expense procedure is currently being sold to dental practices in a complete kit, including bone graft, for $1,000. The cost to the patient for a sinus lift procedure and a dental implant is between $3,000 and $6,000. Maxillent believes that a minimally invasive procedure such as iRaise can command a premium price. After all, time is money, when you consider the extensive downtime and pain associated with an open sinus lift.
Author bio
Tal Moyal is director of sales and marketing at Maxillent Ltd. She has more than 10 years of management experience in multinational dental companies and has managed local and international sales, marketing, and distribution channels. Tal led the development of distributor’s network and personal relationships, increasing bottom line results. She brings a proven track record in developing and implementing sales and marketing strategies, new product launches, creating pricing strategies as well as breaking ground into international markets. Tal holds an MBA in financial management from Manchester University.