Oral gel contains cancer-preventing compounds derived from black raspberries
By Maria Perno Goldie, RDH, MS, with the assistance of Allison Walker
Maria Perno Goldie (MPG): I had the opportunity to interview Dr. Susan Mallery, who is a humble as she is intelligent. I had the assistance of Allison Walker, a freelance journalist who has been involved in dental publishing for more than 20 years.
Maria Perno Goldie (MPG): I had the opportunity to interview Dr. Susan Mallery, who is a humble as she is intelligent. I had the assistance of Allison Walker, a freelance journalist who has been involved in dental publishing for more than 20 years.
Dr. Susan Mallery (SM) is a Professor in the Division of Oral Surgery, Oral Pathology, and Anesthesiology at The Ohio State University, College of Dentistry, in Columbus, Ohio. Her research interests include oral cancer initiation, AIDS-related oral cancer and chemoprevention. Dr. Mallery has published articles in journals such as Cancer Research, Cancer Prevention Research, Molecular Pharmaceutics, Carcinogenesis and Clinical Cancer Research, to name a few. She graduated from The Ohio State University with her DDS and later returned to receive her oral pathology specialty training and a PhD in Pathology. Dr. Mallery is licensed by the Ohio State Dental Board and board certified by the American Board of Oral Pathology and American Academy of Oral Pathology. She belongs to the American Academy of Oral Pathology, American Board of Oral Pathology, American Association for Cancer Research, and is a Fellow of the American Association for the Advancement of Science. She is a consultant at The Ohio State University and James Cancer hospitals. MPG: Oral squamous cell carcinoma (OSCC) will be diagnosed in more than 36,000 Americans this year and has a particularly high mortality rate—as it will kill approximately 8,000 patients this year. As excisional surgery is the primary treatment for OSCC—even those patients who are cured suffer loss of tissues critical for esthetics, speech and eating. Due to OSCC’s high rates of morbidity and mortality and its high socio-economic impact, a strategy to prevent progression of precancerous oral lesions to OSCC is more appealing. Currently, precancerous oral lesions are surgically removed—with either a blade or laser—and the tissues evaluated microscopically. Discouragingly, approximately 30% of lesions that are completely removed as confirmed by microscopic evaluation recur and some progress to OSCC. Dr. Mallery has dedicated her nearly 30-year career to studying new strategies to preventing oral cancer. Her research has been supported by funding from the National Cancer Institute (NCI) of the National Institutes of Health (NIH) and The Ohio State University (OSU) Center for Clinical and Translational Science. It is also funded by the Fanconi Anemia Research Fund, a grassroots organization whose mission is to find effective treatments and a cure for Fanconi anemia and to provide education and support services to affected families worldwide. Dr. Mallery stresses that she is a part of a team, and that the research is truly a team effort.Fanconi anemia (FA) is one of the inherited anemias that leads to bone marrow failure (aplastic anemia). It is a recessive disorder: if both parents carry a defect (mutation) in the same FA gene, each of their children has a 25% chance of inheriting the defective gene from both parents. When this happens, the child will have FA. Fanconi anemia patients have an extremely high risk of developing squamous cell cancers in areas of the body in which cells normally reproduce rapidly, such as the oral cavity, esophagus, the gastrointestinal tract, the anus, and the vulva. FA patients may develop these cancers at a much earlier age than people without Fanconi anemia. Patients who have had a successful bone marrow transplant and, thus, are cured of the blood problems associated with FA, still must have regular examinations to watch for signs of cancer. Head and neck squamous cell carcinoma (HNSCC) is a significant threat for people with FA, regardless of bone marrow transplantation status. Not only is the incidence of HNSCC considerably higher than in the general population (500-700 times higher), patients with FA present with these types of cancers at a younger age than those without FA – the median age is 27 years. Regular screenings are critically important. MPG: Dr. Mallery, you have been investigating a number of agents to identify new therapeutics that can suppress the conversion of pre-cancerous to cancerous cells (chemoprevention), in particular, anthocyanins. Can you explain this to us?
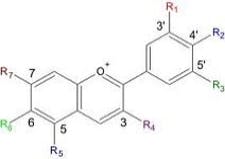
SM: Chemoprevention is a way to prevent or delay the development of cancer by taking medicines, vitamins, or other agents. My colleagues and I are using a bimodal approach. Our first breakthrough was the creation of an oral gel containing high concentrations of anthocyanins, powerful cancer-preventing compounds found in black raspberries. Study results showed that the gel, when applied to the mouth, selectively removed atypical epithelial cells for the population through either preprogrammed cell death (apoptosis) or causing terminal differentiation (making the protective keratin covering). MPG: Can you explain the mechanism of action of these anthocyanins?
SM: As briefly mentioned above, anthocyanins—and likely other black raspberry (BRB) compounds—are capable of modulating epithelial cell growth by affecting intracellular signaling and gene expression. Also apparent from our pilot study was that some patients derived more benefit from gel application. These inter-patient differences prompted a later study to help identify the cause. Analyses of saliva samples collected after BRB rinses were conducted to assess local pharmacokinetics and compare the capacities of three different BRB rinse formulations to provide sustained intraoral levels of anthocyanins. Not surprisingly, these studies showed that BRB metabolism was affected by three intraoral enzymatic components, i.e. (1) oral tissues, (2) saliva, and (3) oral bacteria (“microflora”). As all three components affected BRB bioactivation and local retention, it is likely inter-patient differences in these three areas that contribute in large part to BRB gel responsiveness. We are currently conducting the logical extension study of the pilot trial, which entails inclusion of a gel-placebo and multicenter testing. Results to date have confirmed therapeutic efficacy is limited to the BRB gel formulation and not the placebo. More complex analyses—which assess the gel’s effect at the molecular level—are ongoing. One of the largest challenges with oral cancer chemoprevention is to develop an effective, yet nontoxic strategy. Efficacy speaks for itself—the need for nontoxic is tied to the fact that many to most of these treatments will be necessary for the life of the patient. The lifelong need is tied to the fact that persons who develop precancerous oral lesions have genetic mutations in the cells that are key for future epithelial generations, i.e., epithelial stem cells. When stem cells divide (which is not very often), they make perfect copies of themselves. Consequently, if the stem cells are mutated, their daughter cells faithfully carry forward this mutation. Our BRB gel data imply that locally applied black raspberry constituents can re-direct appropriate epithelial cell growth by removing mutated cells from the overall cell population. Consistent with a food-based approach, no systemic or local toxicities occurred. Local delivery allows for better therapeutic concentration at the site with fewer systemic side effects. With oral cancer chemoprevention given systemically, the liver, in first-pass metabolism, makes the agent less active than the parent compound. The first-pass effect (also known as first-pass metabolism or presystemic metabolism) is a phenomenon of drug metabolism whereby the concentration of a drug is greatly reduced before it reaches the systemic circulation. It is the fraction of lost drug during the process of absorption that is generally related to the liver and gut wall. We must have a compliant patient population with local delivery, it is vital. In some cases the decision involves having multiple biopsies or applying a gel a number of times per day. Not having frequent biopsies can be a good motivator.MPG: Dr. Mallery, you have been investigating alternatives to the surgical removal of pre-cancerous oral lesions. Can you explain what you have found in this area?
SM: We have turned our attention to identifying alternatives to the surgical removal of pre-cancerous lesions. However, we are not “there” yet. Close clinical follow up is critical. If we suspect a malignant lesion, we must first biopsy, and if it is an active lesion, place the gel to prevent recurrence. MPG: Dr. Mallery went on to tell a story about her passion and her work in one of the studies. She related that one third of the patients in the pilot trial were “super” responders, lesions resolved clinically and histologically, and biochemical and molecular markers returned to normal after treatment. There was an intermediate group of about one third, and the last third did not respond in either a negative or positive way. The researchers wanted to determine what caused the “super” responders to react as they did. The study was done with normal, healthy people, and it was found that there is a large difference in variability in enzyme levels to recycle the product. Best responders bioactivate the product and keep it in place for a long time. Enzyme profiles are being done.
SM: Because BRB components alone are insufficient to regress some patients’ precancerous oral lesions, we have decided to introduce a second chemopreventive, the synthetic vitamin A compound, fenretinide. Fenretinide is a “bench” chemopreventive star capable of causing either differentiation or apoptosis in transformed epithelial cells. Previous fenretinide oral cancer chemoprevention trials, which relied on systemic fenretinide delivery, were unsuccessful. Although none of these studies assessed drug levels at the target site, the pill-based delivery format could not even achieve treatment-relevant blood levels. Furthermore, large systemic doses of fenretinide resulted in toxicities including night blindness and changes in blood lipid profiles. The objective of this study was to enhance oral mucosal permeation of fenretinide by co-incorporation of propylene glycol (PG) and menthol in fenretinide/Eudragit RL PO mucoadhesive patches. Fenretinide is an extremely hydrophobic chemopreventive compound with poor tissue permeability. Co-incorporation PG or menthol in fenretinide/Eudragit RL PO patches led to significant ex vivo fenretinide permeation enhancement. Addition of PG above 2.5 wt% in the patch resulted in significant cellular swelling in the buccal mucosal tissues. These alterations were ameliorated by combining both enhancers and reducing the PG level. After buccal administration of patches in rabbits, in vivo permeation of fenretinide across the oral mucosa was greater relative to permeation obtained from the enhancer-free patch. In vitro and in vivo release of fenretinide from the patch was not significantly increased by co-incorporation of permeation enhancers, indicating that mass transfer across the tissue, and not the patch, largely determined the permeation rate control in vivo. As a result of its improved permeation and its lack of deleterious local effects, the mucoadhesive fenretinide patch co-incorporated with 2.5 wt% PG + 5 wt% menthol represents an important step in the further preclinical evaluation of oral site-specific chemoprevention strategies with fenretinide.Fenretinide was studied in pill form, where there was more drug in the blood versus at the site. There were also toxicity problems. My team and I always thought that fenretinide would be a good drug if delivered in a different manner.
SM: We have turned our attention to identifying alternatives to the surgical removal of pre-cancerous lesions. However, we are not “there” yet. Close clinical follow up is critical. If we suspect a malignant lesion, we must first biopsy, and if it is an active lesion, place the gel to prevent recurrence. MPG: Dr. Mallery went on to tell a story about her passion and her work in one of the studies. She related that one third of the patients in the pilot trial were “super” responders, lesions resolved clinically and histologically, and biochemical and molecular markers returned to normal after treatment. There was an intermediate group of about one third, and the last third did not respond in either a negative or positive way. The researchers wanted to determine what caused the “super” responders to react as they did. The study was done with normal, healthy people, and it was found that there is a large difference in variability in enzyme levels to recycle the product. Best responders bioactivate the product and keep it in place for a long time. Enzyme profiles are being done.
SM: Because BRB components alone are insufficient to regress some patients’ precancerous oral lesions, we have decided to introduce a second chemopreventive, the synthetic vitamin A compound, fenretinide. Fenretinide is a “bench” chemopreventive star capable of causing either differentiation or apoptosis in transformed epithelial cells. Previous fenretinide oral cancer chemoprevention trials, which relied on systemic fenretinide delivery, were unsuccessful. Although none of these studies assessed drug levels at the target site, the pill-based delivery format could not even achieve treatment-relevant blood levels. Furthermore, large systemic doses of fenretinide resulted in toxicities including night blindness and changes in blood lipid profiles. The objective of this study was to enhance oral mucosal permeation of fenretinide by co-incorporation of propylene glycol (PG) and menthol in fenretinide/Eudragit RL PO mucoadhesive patches. Fenretinide is an extremely hydrophobic chemopreventive compound with poor tissue permeability. Co-incorporation PG or menthol in fenretinide/Eudragit RL PO patches led to significant ex vivo fenretinide permeation enhancement. Addition of PG above 2.5 wt% in the patch resulted in significant cellular swelling in the buccal mucosal tissues. These alterations were ameliorated by combining both enhancers and reducing the PG level. After buccal administration of patches in rabbits, in vivo permeation of fenretinide across the oral mucosa was greater relative to permeation obtained from the enhancer-free patch. In vitro and in vivo release of fenretinide from the patch was not significantly increased by co-incorporation of permeation enhancers, indicating that mass transfer across the tissue, and not the patch, largely determined the permeation rate control in vivo. As a result of its improved permeation and its lack of deleterious local effects, the mucoadhesive fenretinide patch co-incorporated with 2.5 wt% PG + 5 wt% menthol represents an important step in the further preclinical evaluation of oral site-specific chemoprevention strategies with fenretinide.Fenretinide was studied in pill form, where there was more drug in the blood versus at the site. There were also toxicity problems. My team and I always thought that fenretinide would be a good drug if delivered in a different manner.
I worked with Peter Larsen, DDS, Chair of Division, Oral Maxillofacial Surgeon, Gary Stoner, PhD, and Kashappa Goud Desai, PhD, in both trials. Steven P. Schwendeman, PhD, is a pharmaceutical chemist, Professor and Chair Department of Pharmaceutical Sciences, College of Pharmacy, at the University of Michigan. His lab developed the fenretinide patch with Kashappa-Goud Desai, PhD. Fenretinide is lipophillic, and they needed the formulation to be stable, for the patch to stick on the site, deliver drug, and allow the drug to penetrate in an aqueous environment into keratinized tissue. It is great science! These two researchers are involved with patent application for the fenretinide patch, to be placed on active or recently excised lesions.A combination approach with these two chemotherapeutics may someday be achieved and they may be complementary or synergistic. They have different mechanisms of action and if delivered at the same time could be antagonistic. The dosing must be staggered, with initial application, and perhaps 12 hours later, delivery of the next drug. When the patch is applied to lesions, pharmacokinetic studies show no drug in the saliva. The hypothesis is for targeted delivery and uptake of fenretinide, followed by field coverage with the raspberry rinse. A published study stated that the objective was to develop fenretinide oral mucoadhesive patch formulations and to evaluate their in vitro and in vivo release performance for future site-specific chemoprevention of oral cancer. The gel was used topically at the site of the lesion or after excision. Our goal is to create complementary oral cancer chemoprevention strategies that would permit targeted delivery directly to visible lesions as well as address the need for field coverage throughout the mouth. My colleagues and I are optimistic that optimized delivery formulations and dosing schedules for BRB and fenretinide will help make appreciable clinical progress. We aim to prevent cancerization, which is transformation of cells into cancer, or from a normal to a cancerous state. The concept is that being exposed to toxins and metabolic enzymes can activate toxins and cause the mutated cells to become active. There is now a multi-centered NCI trial of the raspberry product in patients, based on the pilot study. There are two manuscripts, one published and one pharmacokinetic study in rabbits ready to be published. The patch is considered a device by the FDA and they must apply as an Investigational New Device (IND). It is a very safe drug.MPG: Dr. Mallery, what is your advice about prevention of oral cancers?
SM: I recommend not using tobacco in any form, using alcohol in moderation, visiting an oral healthcare provider at least every six months, practicing good oral hygiene, living a healthy lifestyle, having good nutrition, and providing immunization against the human papillomavirus (HPV) for sons and daughters. Precancerous lesions (oral dysplasia) tend to be on the floor of mouth, lateral border of the tongue, etc. The raspberry gel is sticky, and we are trying to get the adherent patch dosage as a “burst delivery” every 15 minutes. Patients are told not to eat or drink for 30 minutes, and the patch is designed for use multiple times throughout the day. It will be a prescription agent. Research has been conducted in Dr. Schwendeman’s lab on oral cancer patients with polyglycolic acid and polylactic acid implants (properties similar to resorbable sutures) that can deliver drug in the former cancer site. We know our patient population, they may not apply something four times a day. Polymeric implants for cancer chemotherapy may be one of the answers.MPG: I want to thank Dr. Mallery for her time and expertise. I also wish to thank Allison Walker for her assistance with this interview.Additional reading
1. www.fanconi.org/index.php/learn_more.2. www.fanconi.org/index.php/learn_more/relationship_to_cancer.3. www.mdanderson.org/patient-and-cancer-information/cancer-information/cancer-topics/prevention-and-screening/chemoprevention/index.html.4. Mallery SR, Budendorf DE, Larsen MP, Pei P, Tong M, Holpuch AS, Larsen PE, Stoner GD, Fields HW, Chan KK, Ling Y, Liu ZEffects of human oral mucosal tissue, saliva, and oral microflora on intraoral metabolism and bioactivation of black raspberry anthocyanins. Cancer Prev Res (Phila) 4(8) 1209-21 8/1/2011.5. en.wikipedia.org/wiki/First_pass_effect. 6. Wu X, Desai KG, Mallery SR, Holpuch AS, Phelps MP, Schwendeman SP. Mucoadhesive Fenretinide Patches for Site-specific Chemoprevention of Oral Cancer: Enhancement of Oral Mucosal Permeation of Fenretinide by Co-incorporation of Propylene Glycol and Menthol. Mol Pharm. 2012 Jan 26. [Epub ahead of print].7. Goodman A. Moving Chemoprevention Forward into the Most Promising Areas of Study. Aerodigestive, Gynecologic, Prostate, & Bladder Cancers. Oncology Times, March 10, 2003 - Volume 25 - Issue 5, p 25-26. 8. Holpuch AS, Desai KH, Schwendeman SP, Mallery SR. Optimizing therapeutic efficacy of chemopreventive agents: A critical review of delivery strategies in oral cancer chemoprevention clinical trials. J Carcinog 2011; 10:23) 9. Desai KH, Mallery SR, Holpuch AS and Schwendeman SP. Development and In Vitro-In Vivo Evaluation of Fenretinide-Loaded Oral Mucoadhesive Patches for Site-Specific Chemoprevention of Oral Cancer. Pharmaceutical Research, Volume 28, Number 10, 2599-2609. (www.springerlink.com/content/h54g2w4835031l5x/)
SM: I recommend not using tobacco in any form, using alcohol in moderation, visiting an oral healthcare provider at least every six months, practicing good oral hygiene, living a healthy lifestyle, having good nutrition, and providing immunization against the human papillomavirus (HPV) for sons and daughters. Precancerous lesions (oral dysplasia) tend to be on the floor of mouth, lateral border of the tongue, etc. The raspberry gel is sticky, and we are trying to get the adherent patch dosage as a “burst delivery” every 15 minutes. Patients are told not to eat or drink for 30 minutes, and the patch is designed for use multiple times throughout the day. It will be a prescription agent. Research has been conducted in Dr. Schwendeman’s lab on oral cancer patients with polyglycolic acid and polylactic acid implants (properties similar to resorbable sutures) that can deliver drug in the former cancer site. We know our patient population, they may not apply something four times a day. Polymeric implants for cancer chemotherapy may be one of the answers.MPG: I want to thank Dr. Mallery for her time and expertise. I also wish to thank Allison Walker for her assistance with this interview.Additional reading
1. www.fanconi.org/index.php/learn_more.2. www.fanconi.org/index.php/learn_more/relationship_to_cancer.3. www.mdanderson.org/patient-and-cancer-information/cancer-information/cancer-topics/prevention-and-screening/chemoprevention/index.html.4. Mallery SR, Budendorf DE, Larsen MP, Pei P, Tong M, Holpuch AS, Larsen PE, Stoner GD, Fields HW, Chan KK, Ling Y, Liu ZEffects of human oral mucosal tissue, saliva, and oral microflora on intraoral metabolism and bioactivation of black raspberry anthocyanins. Cancer Prev Res (Phila) 4(8) 1209-21 8/1/2011.5. en.wikipedia.org/wiki/First_pass_effect. 6. Wu X, Desai KG, Mallery SR, Holpuch AS, Phelps MP, Schwendeman SP. Mucoadhesive Fenretinide Patches for Site-specific Chemoprevention of Oral Cancer: Enhancement of Oral Mucosal Permeation of Fenretinide by Co-incorporation of Propylene Glycol and Menthol. Mol Pharm. 2012 Jan 26. [Epub ahead of print].7. Goodman A. Moving Chemoprevention Forward into the Most Promising Areas of Study. Aerodigestive, Gynecologic, Prostate, & Bladder Cancers. Oncology Times, March 10, 2003 - Volume 25 - Issue 5, p 25-26. 8. Holpuch AS, Desai KH, Schwendeman SP, Mallery SR. Optimizing therapeutic efficacy of chemopreventive agents: A critical review of delivery strategies in oral cancer chemoprevention clinical trials. J Carcinog 2011; 10:23) 9. Desai KH, Mallery SR, Holpuch AS and Schwendeman SP. Development and In Vitro-In Vivo Evaluation of Fenretinide-Loaded Oral Mucoadhesive Patches for Site-Specific Chemoprevention of Oral Cancer. Pharmaceutical Research, Volume 28, Number 10, 2599-2609. (www.springerlink.com/content/h54g2w4835031l5x/)
Maria Perno Goldie, RDH, MS
To read previous articles in RDH eVillage FOCUS written by Maria Perno Goldie, go to articles.