Role of saliva in prevention of caries in exposure to ACBs
By Madhulika Advani, DMD; Joel Mark, DDS; and Zeena AdvaniAbstractThe protective role of saliva has been observed in clinical situations where decreased saliva flow has led to a marked increase in dental caries.1,4 This study sought to assess the role of saliva in prevention of caries and enamel erosion in human teeth, with exposure to acidulated carbonic beverages (ACBs). Seventeen extracted mandibular and maxillary third molars were used in a two-trial study, each trial consisting of eight molars. The teeth were exposed to four different ACBs — Sprite, Mountain Dew, Coca-Cola, and Gatorade. Two teeth were assigned to each ACB per trial, one of which also contained saliva. Over a period of seven days, pH and digital radiographic changes were assessed, followed by morphological observations of surface texture of the teeth. Changes in the digital images were a reflection of the mineral lost due to ACBs. Images were compared with respect to teeth immersed in saliva; final results indicated less mineral loss on the external and internal surfaces of the specimens when exposed to saliva.
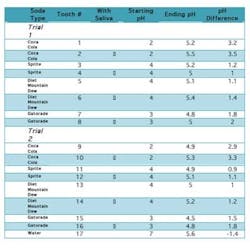
IntroductionThe consumption of carbonated beverages has increased dramatically over recent years. Across the globe dental professionals have faced a rapid increase in caries, recurring decay, and dental erosions. This has led to a huge increase in the cost involved in maintaining dental health — a direct result of augmented ACB intake. Even though all dentitions are susceptible to demineralization caused by ACBs, the presence of saliva as a buffering agent may reduce the deleterious effect of these beverages.Some of the common ingredients of ACBs are malic, phosphoric, and ascorbic acid; all of which cause extensive enamel/dentin damage and lead to irreversible loss of tooth structure when ingested.2,5,6,7,8 The frequency of consumption leads to an increase in the rate of the decay process; however, the rate is specifically determined by the acidity of the drink consumed. Often drinks are not entirely similar in their contents, thus a difference in their effects on tooth enamel. In this study it was found that Coca-Cola was the most acidic, with a pH of 2, followed by Gatorade (pH 3), Sprite (pH 4), and Diet Mountain Dew (pH 4). The acidity was clearly reflected in the results obtained: the molars exposed to ACBs of lower pH levels were found to have greater surface pitting, decalcification, and mottling than the others. Digital X-rays demonstrated higher levels of decalcification and demineralization internally as well.The effects of acidity can be minimized with the addition of saliva. Its secretion is essential in the prevention of dental caries and infection; it serves to remove food debris, dead cells, bacteria, and white blood cells while neutralizing damaging fluids and acids. These tasks are diminished when salivary secretion decreases. At this point the acidity in the mouth increases, contributing to an increase in decay. ObjectivesThe purpose of this investigation was to observe the effect of saliva in protecting dental tissues from enamel erosion caused by ACBs. The study took place over a period of seven days of prolonged exposure of ACBs, the effects of which are equivalent to 6.5 years of daily ACB consumption.3 The changes noted were observed radiographically along with visual and photographic aids. Materials and methodsSeventeen maxillary and mandibular human molars were utilized in this investigation. The teeth were recently extracted and stored in Cidex PA (Advanced Sterilization Products, Irvine, Calif.) for two weeks. Each was chosen from a different patient at random and was cleaned of any deposits and debris before use. All molars were intact and free from caries at the time of the extraction.Before beginning, initial observations were recorded using digital radiographs (Schick Technologies, GX-770). A standardized positioning device was placed and a paralleling technique was used to take the radiographs; radiation exposure was set at 70 kVp, 7 mA, and 11 impulses. Radiographic images were taken of the crown and root in particular. Radiographic images were colorized in order to observe mineral density, which would become a vital evaluation in noting internal changes within the molars. Photographs were also taken of the teeth (CDR 2000 Interface, Schick Technologies, Intraoral Camera size 2); topography and morphology of the teeth were noted prior to the exposure of ACBs to establish an initial baseline. Both were used throughout the investigation to assess the changes in enamel surface texture, luster, and internal demineralization. Initial examination of the specimens further included an account of mass. Each molar was weighed on a scale and recorded in grams. The experiment was designed and conducted in the following manner: Two trials were conducted in this experiment; consequently the teeth were separated into two groups of eight. One tooth was set aside to be used as the control of the experiment. Each tooth was X-rayed individually using digital radiography to observe the layers of the teeth. The images were colorized to observe the mineral density.Following radiographic scans, each tooth was placed in its own container and its respective liquid — either Coca-Cola (pH 2), Sprite (pH 4), Diet Mountain Dew (pH 4), or Gatorade (pH 3). For both trials (each trial consisting of eight molars), two molars were exposed to one type of liquid, with the 17th molar serving as a control and placed in tap water (pH 7, 12.4°C). Each molar was placed in 78.8 mL of fluid.Along with its specified liquid, every second tooth was placed in a container with 1.5 mL of saliva (average stimulated salivary flow per minute),1 used from the same individual. The method allowed for observation of saliva’s protective effects; one molar was exposed only to a liquid while the second was exposed to the same liquid in addition to saliva. Observations during the course of the investigation were made at increasing intervals. The pH and topographic features of each molar were initially noted after 30 minutes, one hour, two hours, six hours, 12 hours, and 24 hours. Factors were checked every 24 hours thereafter. During observations, liquid contents such as bacterial formation, froth, and debris particles were also recorded. After seven days, final interpretations were made, and the teeth were removed from their containers. Each was rinsed, dried, and weighed again on the scale. All molars were also radiographed and photographed again, with the mineralization of each tooth and thickness of the enamel observed. Photographs were used to compare the external texture and appearance of the control molar to those exposed to ACBs. ResultsExternal appearanceAt the beginning of this experiment for both trials, the appearance of the teeth was noted. The enamel of the extracted teeth was intact and cavity-free. The surface seemed smooth and glossy. This enamel had not been exposed to the damaging effects of acidic drinks, and there was no evidence of pitting or surface decalcification of a decaying tooth. After one week, the teeth being exposed to only the drinks were observed to have a chalky surface, pitted, and a complete loss of surface glossiness. This indicated severe damage to the enamel, and the teeth began to display early signs of decay and demineralization of hydroxyapatite. The teeth placed in saliva and the drinks did exhibit similar damage, but to a lesser extent. There was less pitting, and some areas of the enamel retained its glossy appearance. In both cases where the teeth were exposed to drinks with and without saliva, there was absorption of the color. For example, those teeth placed in Mountain Dew had roots that were completely yellow. The most dramatic color change was seen in Coke, in which both the enamel and roots seemed to be affected. Radiographic observationsX-rays of the teeth show that the enamel is seen to be most dense, followed by the dentin. The pulp chamber was observed to be the least dense. After the seven-day period, the teeth that were placed only in the beverages seemed to have undergone a dramatic loss of mineral, making the teeth appear more porous and less dense. Those teeth placed in drinks and saliva seemed to have less loss of mineral and a much higher density as seen radiographically.pH changesAll of the drinks used in the experiment were extremely acidic, the most being Coca-Cola with a pH of 2. Even in the presence of saliva, the pH did not change after 48 hours. The pH only changed to become less acidic after seven days of the experiment, possibly neutralized by the loss of mineral from the tooth.
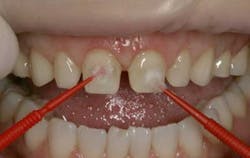
Occlusal view of teeth following experiment (clockwise): Tooth No.1 (without saliva), Control, Tooth No. 2 (with saliva)
Occlusal view of teeth following experiment (clockwise): Control, Tooth No. 5 (without saliva), Tooth No. 6 (with saliva)
Comparison of Tooth No. 10 (exposure to Coca-Cola with saliva) to Control (left)
Comparison of Tooth No. 9 (exposure to Coca-Cola without saliva) to Control (left)
Comparison of Tooth No. 14 (exposure to Diet Mountain Dew with saliva) to Control (left)
Comparison of Tooth No. 13 (exposure to Diet Mountain Dew without saliva) to Control (left)Drinks comparisonOut of the four liquids used in this experiment, Coca-Cola was the most acidic with a pH of 2 and had the worst effect on the teeth, causing significant pitting and erosion. It was followed by Gatorade with a pH of 3, and Mountain Dew and Sprite with a pH of 4. These drinks showed less decalcification and pitting, respectively. ConclusionAfter performing two trials of this experiment, one can draw the conclusion that saliva does in fact have a protective effect on teeth. When exposed to highly acidic drinks such as Coca-Cola, the saliva was able to prevent a great amount of pitting and decalcification from occurring. The importance of salivary protection in preventing tooth decay cannot be overemphasized. From a clinical standpoint this is a very critical observation, for when saliva production is reduced due to xerostomia (dry mouth) or even daily variations of salivary flow, teeth are left unprotected and can start decaying at a much faster rate than if saliva were present.1,9,10 The advice clinicians can give to patients, especially teenagers, is to completely avoid acidic drinks, especially at night-time when there is a drastic reduction in the salivary flow. This also applies to medication-induced xerostomia, in an already aging population where the rate of caries is rapidly increasing. When there is a great amount of tooth decay as a result of little saliva, patients experience pain and suffering, inability to chew properly, and an increase in health costs. The best advice to give to patients is to refrain from consuming ACBs on a regular basis because they can cause great harm and damage to their dentition.
Madhulika Advani, DMD, is a practicing general dentist in Rye Brook, N.Y. She graduated from the University of London in 1982 with a BDS in dentistry, and has an LDS from the Royal College of Surgeons in England. In 1986 she received a DMD degree from the University of Mississippi School of Dentistry, and in 1990 she completed her General Practice Residency at the Metropolitan Hospital in New York City. As a solo practitioner, Dr. Advani has 20 years of experience in all phases of dentistry. Providing comprehensive patient care is of utmost importance to her, including patient education and service to the community.
Joel Mark, DDS, graduated from NYU College of Dentistry in 1978. Prior to studying at NYU College of Dentistry, he attended SUNY at Buffalo. Dr. Mark has been practicing for more than 28 years. He has been in his current location for 21 years. Since he began practicing dentistry, Dr. Mark has kept up to date on many continuing educational classes, ensuring that his patients receive the most comprehensive and advanced dental care. Currently, Dr. Mark is a senior advisor and guest lecturer at the NYU College of Nursing at NYU College of Dentistry.
Zeena Advani is a senior at Westhill High School, interested in pursuing a career in the medical field. She began a clinical research project for her high school, and selected the topic. She designed the experiment and controls as well as documented the research under guidance of both Dr. Advani and Dr. Mark. Zeena presented the results at the Science Fair, receiving first place for a well-designed and informative project. References1 Perno JL, Crossley H. Unraveling the mysteries of saliva: its importance in maintaining oral health. General Dentistry 2007; 55.4:288-96.2 Bassiouny MA, Kuroda S, Yang J. Topographic and radiographic profile assessment of dental erosion. General Dentistry 2007; 55.4:297-305.3 Von Fraunhofer A, Matthew RM. Effects of sports drinks and other beverages on dental enamel. General Dentistry 2005; 53.1:28-31.4 Thanabodi P, Chanothai, Hengtrakool, et al. The effect of salivary factors on dental erosion in various age groups and tooth surfaces. The Journal of the American Dental Association 2009; 140.9:1137-143.5 Jensdottir T, Nauntofte B, Buchwald C, et al. Effects of sucking acidic candy on whole-mouth saliva composition. Caries Research 2005; 39.6:468-74.6 Larsen MJ, Nyvad B. Enamel erosion by some soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Caries Research 1999; 33:81-87.7 Bassiouny MA, Kuroda S, Yang J. Topographic and radiographic profile assessment of dental erosion Part II: effect of citrus fruit juices on human dentition. General Dentistry 2008;56.2:136-43.8 Squires S. Why soft drinks are dangerous to your health. Natural Health: Chet Day's Huge Collection of Healthy Eating Recipes and Natural Health Articles. Web. 19 Mar. 2008. http://chetday.com/softdrinkdangers.htm.9 Wetton S, Hughes J, West N, et al. Exposure time of enamel and dentine to saliva for protection against erosion: a study in vitro. Caries Research 2006; 40.3:213-17.10 Hara AT, Ando M, González-Cabezas C, et al. Protective effect of the dental pellicle against erosive challenges in situ. Journal of Dental Research 2006; 85:612-16.